Preparation method of ammonium polyphosphate flame retardation material
A technology of ammonium polyphosphate and flame retardant materials, applied in the field of flame retardants, which can solve the problems of high hygroscopicity and low degree of polymerization, and achieve the effects of easy separation, high degree of polymerization, and easy regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
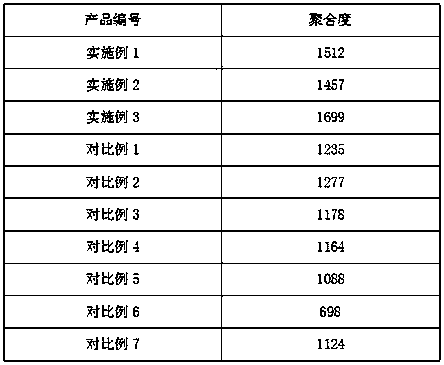
Examples
Embodiment 1
[0016] 1. Resin supported catalyst
[0017] In parts by weight, 1000 g of flake-shaped porous polyimide resin, 0.5 g of 1-n-butyl-3-methylimidazolium hexafluorophosphate, (2-carboxyethyl) triphenyltribromo Phosphine 5g, 2,2-bis(4-carboxyphenyl)hexafluoropropane 100g, 2,2'-bipyridine-4,4'-dicarboxylic acid 25g, europium trifluoroacetate 35g, heat up to 85°C, mix 5h, the resin catalyst product was obtained.
[0018] 2. Melt Polymerized Ammonium Polyphosphate
[0019] In parts by weight, add 10Kg of diammonium hydrogen phosphate, 9Kg of phosphorus pentoxide, 2.25Kg of urea, 0.2Kg of type II ammonium polyphosphate, and 0.3Kg of resin catalyst into the reactor, heat up to 200°C, and react for 4 hours. The resin catalyst is separated, and the product is filtered, washed, dried and pulverized to obtain the ammonium polyphosphate flame retardant material.
Embodiment 2
[0021] 1. Resin supported catalyst
[0022] In parts by weight, 1000 g of flake-shaped porous polyimide resin, 0.1 g of 1-n-butyl-3-methylimidazolium hexafluorophosphate, (2-carboxyethyl) triphenyltribromo Phosphine 1g, 2,2-bis(4-carboxyphenyl)hexafluoropropane 50g, 2,2'-bipyridine-4,4'-dicarboxylic acid 10g, europium trifluoroacetate 20g, heat up to 75°C, mix 4h, the resin catalyst product was obtained.
[0023] 2. Melt Polymerized Ammonium Polyphosphate
[0024] In parts by weight, add 10Kg of diammonium hydrogen phosphate, 8Kg of phosphorus pentoxide, 1.5Kg of urea, 0.1Kg of type II ammonium polyphosphate, and 0.2Kg of resin catalyst into the reactor, heat up to 180°C, and react for 4 hours. The resin catalyst is separated, and the product is filtered, washed, dried and pulverized to obtain the ammonium polyphosphate flame retardant material.
Embodiment 3
[0026] 1. Resin supported catalyst
[0027] In parts by weight, 1000 g of flake-shaped porous polyimide resin, 1 g of 1-n-butyl-3-methylimidazolium hexafluorophosphate, (2-carboxyethyl) triphenyl tribromide Phosphine 10g, 2,2-bis(4-carboxyphenyl)hexafluoropropane 150g, 2,2'-bipyridine-4,4'-dicarboxylic acid 50g, europium trifluoroacetate 50g, heat up to 95°C, mix for 6h , to obtain the resin catalyst product.
[0028] 2. Melt Polymerized Ammonium Polyphosphate
[0029] In parts by weight, add 10Kg of diammonium hydrogen phosphate, 10Kg of phosphorus pentoxide, 3Kg of urea, 0.3Kg of type II ammonium polyphosphate, and 0.4Kg of resin catalyst into the reactor, heat up to 220°C, react for 6 hours, and separate after the reaction The resin catalyst is extracted, and the product is filtered, washed, dried, and pulverized to obtain ammonium polyphosphate flame-retardant material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com