Mycoplasma pneumoniae recombinant antigen and its application
A technology of Mycoplasma pneumoniae and recombinant antigen, applied in the biological field, can solve the problems of high price of natural antigen, cumbersome steps of natural antigen, indeterminate yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] In addition, the present application also provides a method for preparing a Mycoplasma pneumoniae recombinant antigen according to an embodiment, comprising the following steps:
[0052] Construct the gene expression sequence of the recombinant antigen of Mycoplasma pneumoniae, optimize its codons, and select the codons preferred by Escherichia coli. The final gene sequence is obtained by chemical synthesis, and the upstream primers are introduced into the BamHI site, and the downstream primers are introduced into The EcoRI site and the coding sequence of 6 His amino acids and the stop codon TAA before the EcoRI site, the specific amino acid sequence of the Mycoplasma pneumoniae recombinant antigen is shown in SEQ ID NO1. After synthesizing the target fragment, use the upstream and downstream primers for PCR amplification. After the amplified PCR fragment is recovered, it is digested with BamHI and EcoRI, and connected to the expression vector pET-32a digested with BamHI...
Embodiment 1
[0071] 1. Construction of Mycoplasma pneumoniae Recombinant Antigen Expression Plasmid
[0072] First, the sequence of the Mycoplasma pneumoniae gene was collected in Genbank, the gene data of Mycoplasma pneumoniae was established, and it was analyzed by computer software to obtain the Mycoplasma pneumoniae adhesion protein P1 (GenBank: AAB95661.1) and adhesion protein P30 (NCBI Reference Sequence : NP_110141.1) the segment with the most detection activity, the segment with the most detection activity of the P1 protein (the 1219th amino acid segment to the 1422th amino acid segment, referred to as the P1A segment, the 1583rd amino acid segment to the 1627th amino acid segment segment, referred to as P1B), and the segment with the most detectable activity of the P30 protein (the 168th amino acid to the 274th amino acid, referred to as the P30 segment) is connected in series through the connecting peptide GGGGS, and the series sequence from the C-terminal to the N-terminal is P1A...
Embodiment 2
[0094] The preparation of embodiment 2 Mycoplasma pneumoniae antibody colloidal gold detection test paper
[0095] 1. Preparation of coated membrane (nitrocellulose membrane)
[0096] Preparation of coating buffer: 6% methanol, 0.01M pH7.2 PBS buffer as coating buffer, filtered through 0.22 μm membrane, set at 4°C for later use, valid for one week.
[0097] 1000mL 0.01M pH 7.2 PBS buffer solution with 6% methanol: 8g NaCL, 0.2g KCL, 2.9g Na2HPO4·12H2O, 0.2g KH2PO4, 60mL methanol, distilled deionized water to 1000mL.
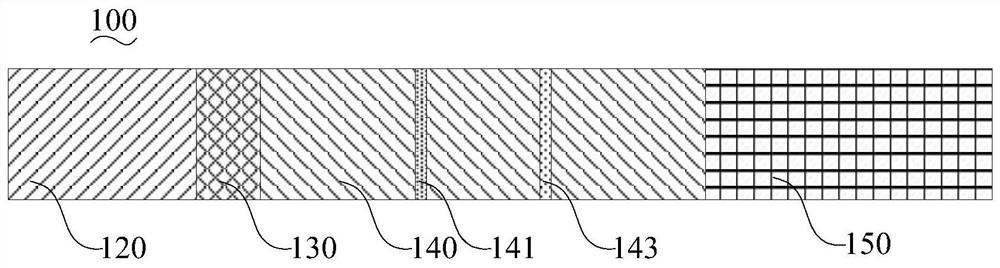
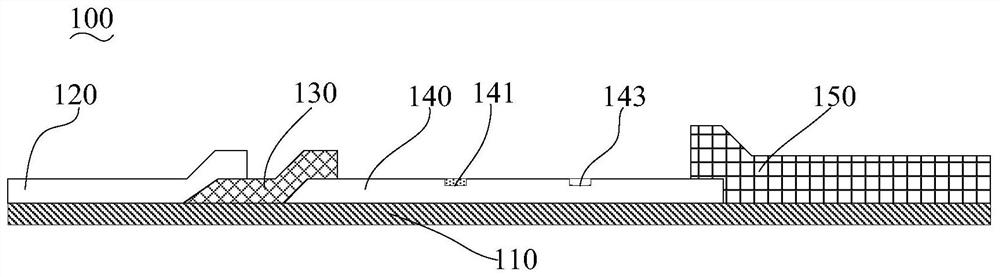
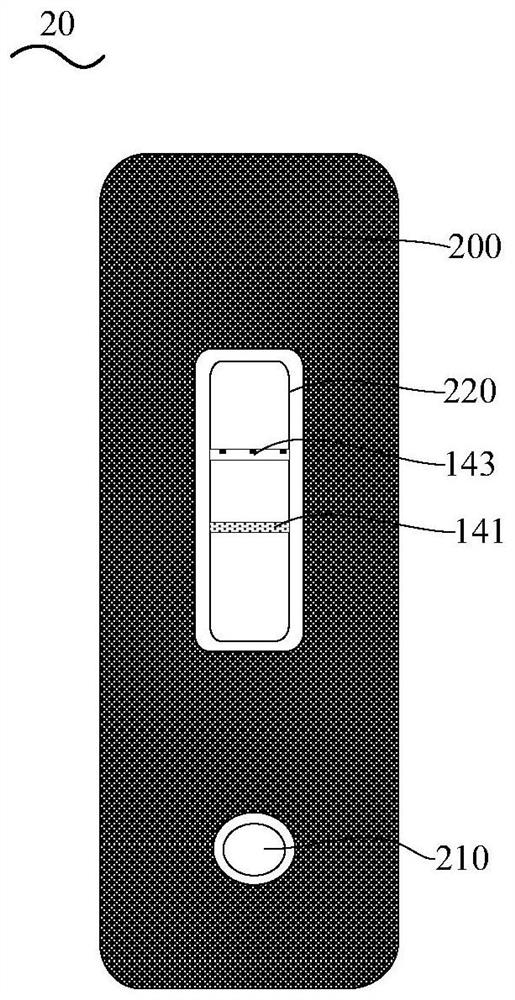
[0098]Preparation of the coated membrane (nitrocellulose membrane): Dilute the Mycoplasma pneumoniae recombinant coated antigen MP-2 to 1-2 mg / mL with the coating buffer, adjust the machine, and draw a line on the detection area of the coated membrane, which is Mycoplasma pneumoniae Antibody detection line (T line). Dilute the rabbit anti-mouse IgG antibody (manufactured by Feipeng Biological Co., Ltd., product number BA-PAB-MU0002) to 1-5 mg / mL with coating ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com