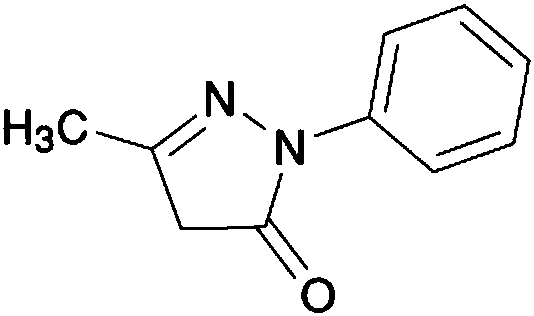
Stable large volume parenteral edaravone injection and preparation process thereof
A technology for edaravone and injection, which is applied to the field of large infusion edaravone injection and its preparation, and can solve the problems of being easily oxidized, not being directly administered, and unavoidable main drug oxidation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
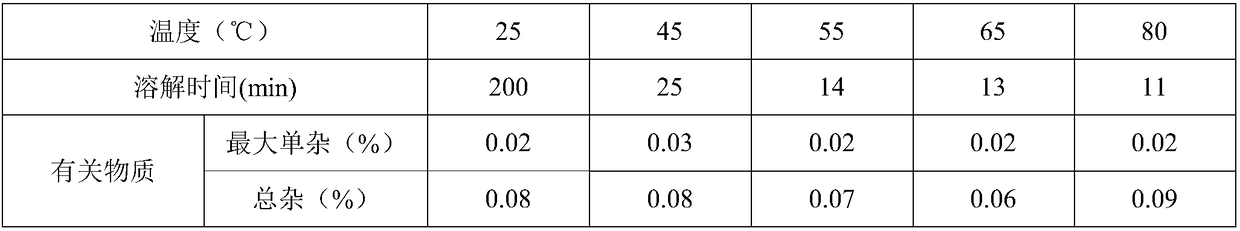
[0031] Embodiment 1: Bulk drug dissolution rate under different temperature conditions
[0032] The research results of the solubility of raw materials show that the solubility of edaravone raw materials in water is poor, and the dissolution rate is relatively slow. It can be considered to increase the dissolution rate of raw materials by increasing the water temperature during dissolution. The specific operation is as follows: Add 30 mg of Edaravone into 100 ml of water at 25°C, 45°C, 55°C, 65°C, and 80°C, seal it, stir until it is completely dissolved, record the dissolution time, and detect related substances; See Table 1.
[0033] Table 1. The dissolution rate of raw materials and related substances at different temperatures
[0034]
[0035] It is known that the raw materials are easily oxidized, so the time for dissolving the raw materials should be reduced as much as possible, taking into account the changes in the related substances of the raw materials after disso...
Embodiment 2
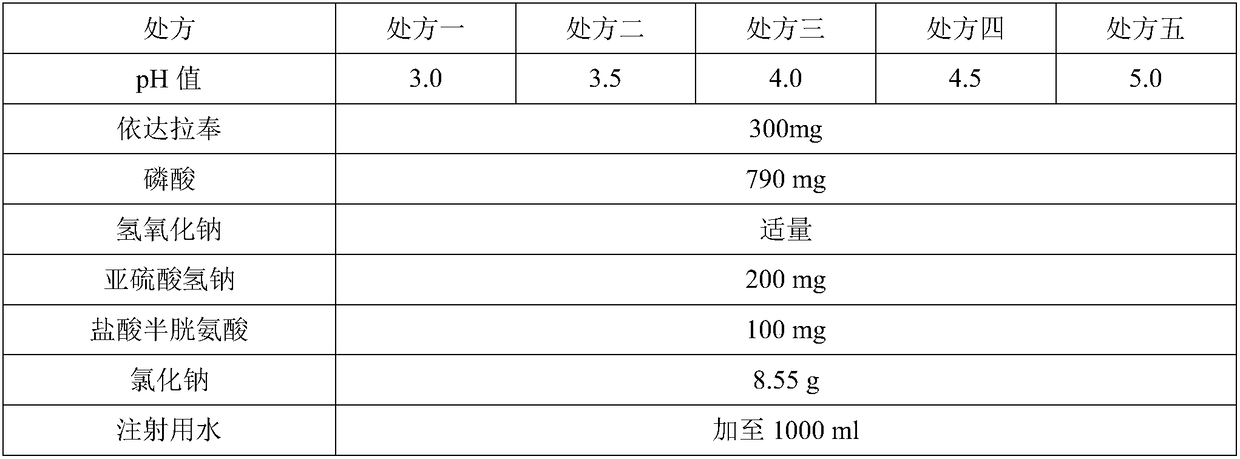
[0036] Embodiment 2: pH value screening
[0037] For injections, the stability of the solution can only be guaranteed under suitable pH conditions, thereby ensuring the safety and effectiveness of the injections. Therefore, the choice of the pH range of the finished product for injection is particularly important and is one of its key quality attributes.
[0038] Table 2. Formulation of the formulation
[0039]
[0040]Preparation Process:
[0041] Dissolve the prescribed amount of sodium chloride with 20-40% water for injection, add 0.05% medicinal charcoal in total, heat to boil and keep boiling slightly for 15 minutes, then cool down to 60-70°C. Stand for 20min, decarbonize. Transfer the filtrate to 50-70% water for injection, boil, cool to 60-70°C, fill with high-purity nitrogen for full protection, add pH regulator, dissolution aid, antioxidant L-cysteine hydrochloride, and the prescription amount in sequence After stirring the edaravone until it completely disso...
Embodiment 3
[0045] Embodiment 3: Screening of pH regulator
[0046] Table 4. Acid Regulator Screening Test Prescription
[0047]
[0048]
[0049] Table 5. Acid regulator screening test results
[0050]
[0051] Prescription 1 (phosphoric acid) and prescription 2 (hydrochloric acid), after sterilization and after 10 days of stability setting out, the appearance of the sample has no obvious change, and the related substances are at a low level, and there is no obvious difference. However, considering hydrochloric acid corrosion equipment, phosphoric acid is preferred.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com