Application of glycerinum-3-phosphoric acid and adjuvant containing glycerinum-3-phosphoric acid and vaccine agent
A technology of glycerol and phosphoric acid, which is applied in the field of immunology, can solve the problems of high price and insignificant immune response effect, and achieve the effects of low cost, improved immune response level and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The Hepatitis A vaccine containing glycerol-3-phosphate adjuvant provided in this example is: after dissolving 2 μl glycerol-3-phosphate with 54 μl normal saline, a solution with a content of 1 mg glycerol-3-phosphate adjuvant is obtained, and the adjuvant is routinely added every A single portion of HAV antigen (that is, a single injection dose used in animal experiments), and finally add physiological saline (PH=7.4) to 300 μL, and mix evenly to obtain a hepatitis A vaccine containing glycerol-3-phosphate.
[0021] Glycerol-3-phosphate, also known as glycerol phosphate, molecular formula: C 3 h 9 o 6 P, molecular weight: 172.07, purchased from J&K, CASNO.57-03-4; live attenuated hepatitis A vaccine, antigen titer 18EU / ml, purchased from Institute of Medical Biology, Chinese Academy of Medical Sciences, Peking Union Medical College.
[0022] Gained immunity test and the effect of the hepatitis A vaccine containing glycerol-3-phosphate adjuvant are as follows:
[002...
Embodiment 2
[0038] The Hepatitis A vaccine containing glycerol-3-phosphate adjuvant provided in this example is: after dissolving 4 μl glycerol-3-phosphate with 54 μl normal saline, a solution with a content of 2 mg glycerol-3-phosphate adjuvant is obtained, and the adjuvant is routinely added every A single portion of HAV antigen (that is, a single injection dose used in animal experiments), and finally add physiological saline (PH=7.4) to 300 μL, and mix evenly to obtain a hepatitis A vaccine containing glycerol-3-phosphate. Wherein the aluminum hydroxide adjuvant and HAV antigen are obtained in the same manner as in Example 1.
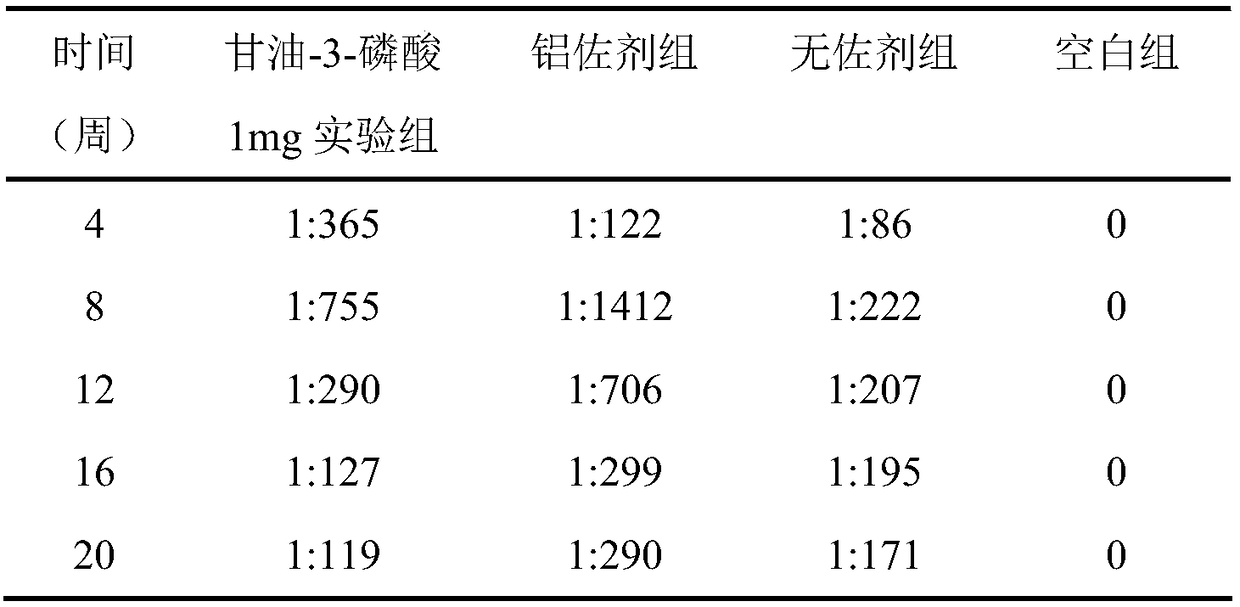
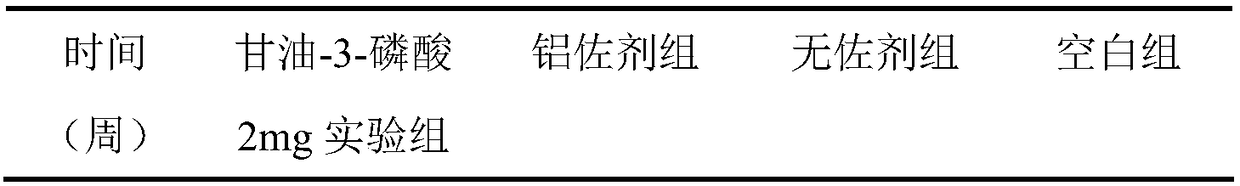
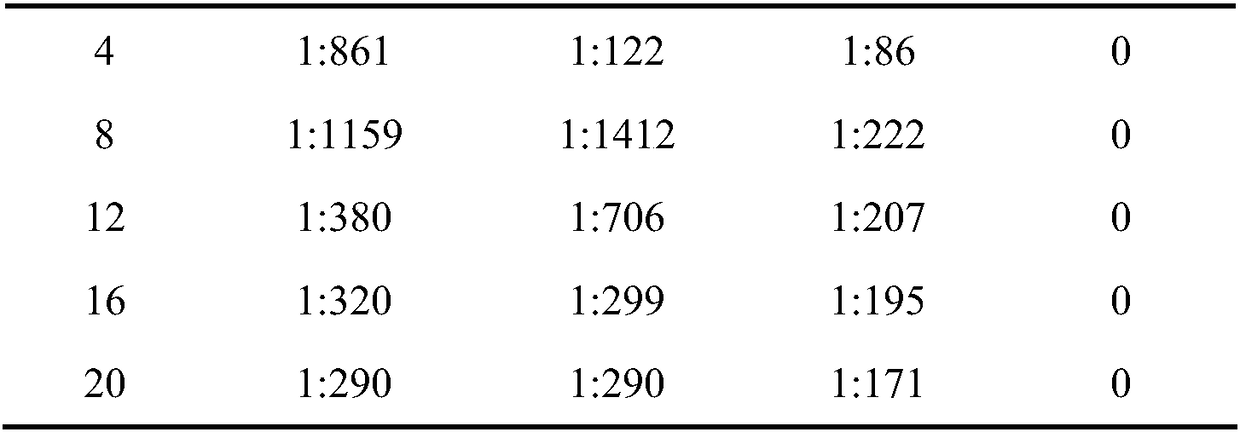
[0039] The immune test of the hepatitis A vaccine containing glycerol-3-phosphate adjuvant obtained in this example is the same as in Example 1, and the results are shown in Table 2. Table 2 shows the serum anti-HAV IgG antibody levels of mice in each experimental group within 20 weeks after using the adjuvant provided in Example 2.
[0040] Table 2
[0041] ...
Embodiment 3
[0045]The Hepatitis A vaccine containing glycerol-3-phosphate adjuvant provided in this example is: after dissolving 8 μl glycerol-3-phosphate with 54 μl normal saline, a solution with a content of 4 mg glycerol-3-phosphate adjuvant is obtained. A single portion of HAV antigen (that is, a single injection dose used in animal experiments), and finally add physiological saline (PH=7.4) to 300 μL, and mix evenly to obtain a hepatitis A vaccine containing glycerol-3-phosphate. Wherein the aluminum hydroxide adjuvant and HAV antigen are obtained in the same manner as in Example 1.
[0046] The immune test of the hepatitis A vaccine containing glycerol-3-phosphate adjuvant obtained in this example is the same as that in Example 1, and the results are shown in Table 3. Table 3 shows the serum anti-HAV IgG antibody levels of mice in each experimental group within 20 weeks after using the adjuvant provided in Example 3.
[0047] table 3
[0048]
[0049] Through data analysis, it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com