Lithium ion battery nonaqueous electrolyte and lithium ion battery
A non-aqueous electrolyte, lithium-ion battery technology, applied in the field of lithium-ion batteries, can solve the problems of easy leakage, expansion of negative electrode material layer, combustion and explosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of non-aqueous electrolyte:
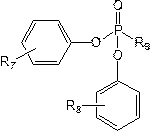
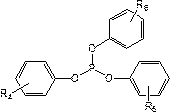
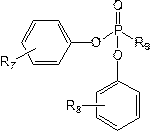
[0047] Configure the electrolyte in the glove box, control the oxygen content in the glove box<2ppm, fill the glove box with nitrogen and control the purity of nitrogen in the glove box to be 99.999%, take 30g ethylene carbonate, 30g diethyl carbonate, 30g carbonic acid Ethyl methyl ester, 2g of fluoroethylene carbonate (FEC), 2g of vinylene carbonate (VC) are mixed, then 15g of lithium hexafluorophosphate and 10g of tris (4-trifluoroethylcarboxyphenyl) phosphate are added and mixed evenly to prepare Non-aqueous electrolyte, denoted as C1;
[0048] (2) Preparation of lithium ion battery:
[0049] Will LiNi 0.5 co 0.2 mn 0.3 o 2 , acetylene black, and polyvinylidene fluoride are mixed uniformly with the positive electrode solvent N-methylpyrrolidone at a weight ratio of 85:10:5 to prepare a positive electrode slurry, which is then coated on an aluminum foil, dried and rolled to prepare a positive electrode sheet; Graphi...
Embodiment 2
[0051] Adopt the same method as Example 1 to prepare non-aqueous electrolyte and lithium ion battery, the difference is that in step (1), 10g of tris (4-trifluoroethoxyphenyl) phosphate is used instead of tris (4-trifluoroethoxyphenyl) phosphate Fluoroethylcarboxyphenyl) phosphate to prepare non-aqueous electrolyte solution C2 and lithium-ion battery S2.
Embodiment 3
[0053] Adopt the method identical with embodiment 1 to prepare non-aqueous electrolytic solution and lithium ion battery, difference is, adopt 10g three (4-trifluoroethoxyphenyl) phosphites to replace three (4- Trifluoroethylcarboxyphenyl) phosphate to prepare non-aqueous electrolyte solution C3 and lithium-ion battery S3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com