Nutritional composition capable of promoting wound healing, bedsore restoration, and postoperative stress ulcer healing
A technology of stress ulcer and nutritional composition, which is applied in the field of decubitus repair, nutritional composition for postoperative stress ulcer healing, promotion of wound healing, and can solve the problem of difficult wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation method of composition of the present invention
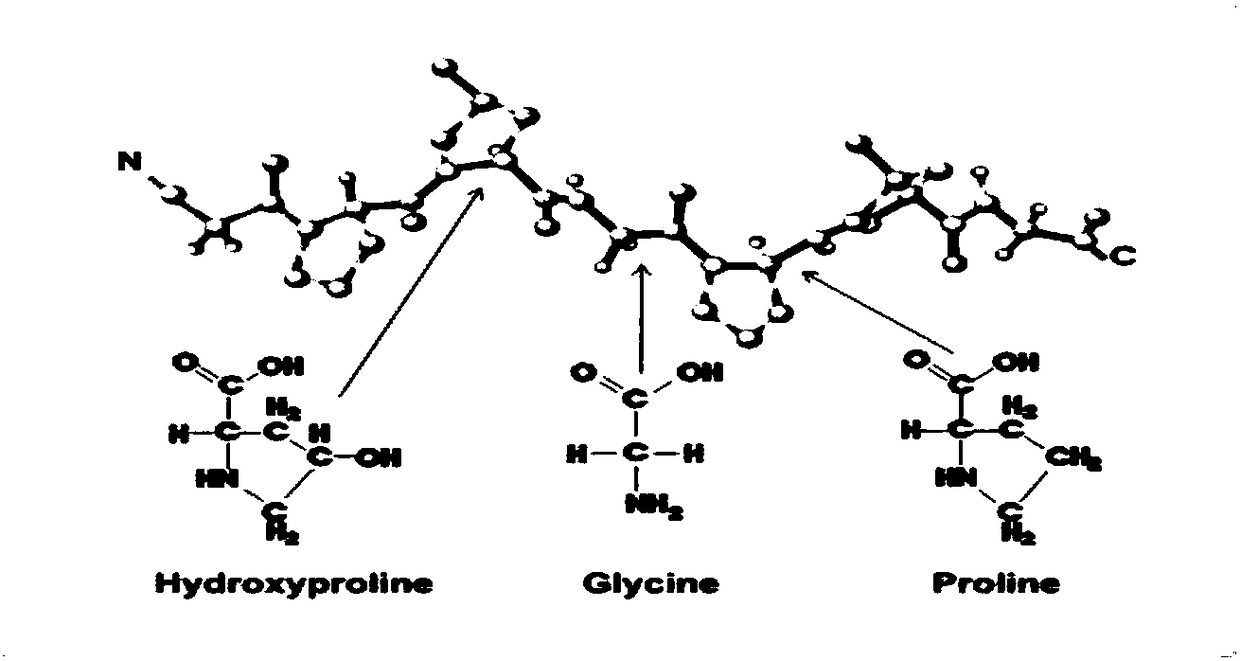
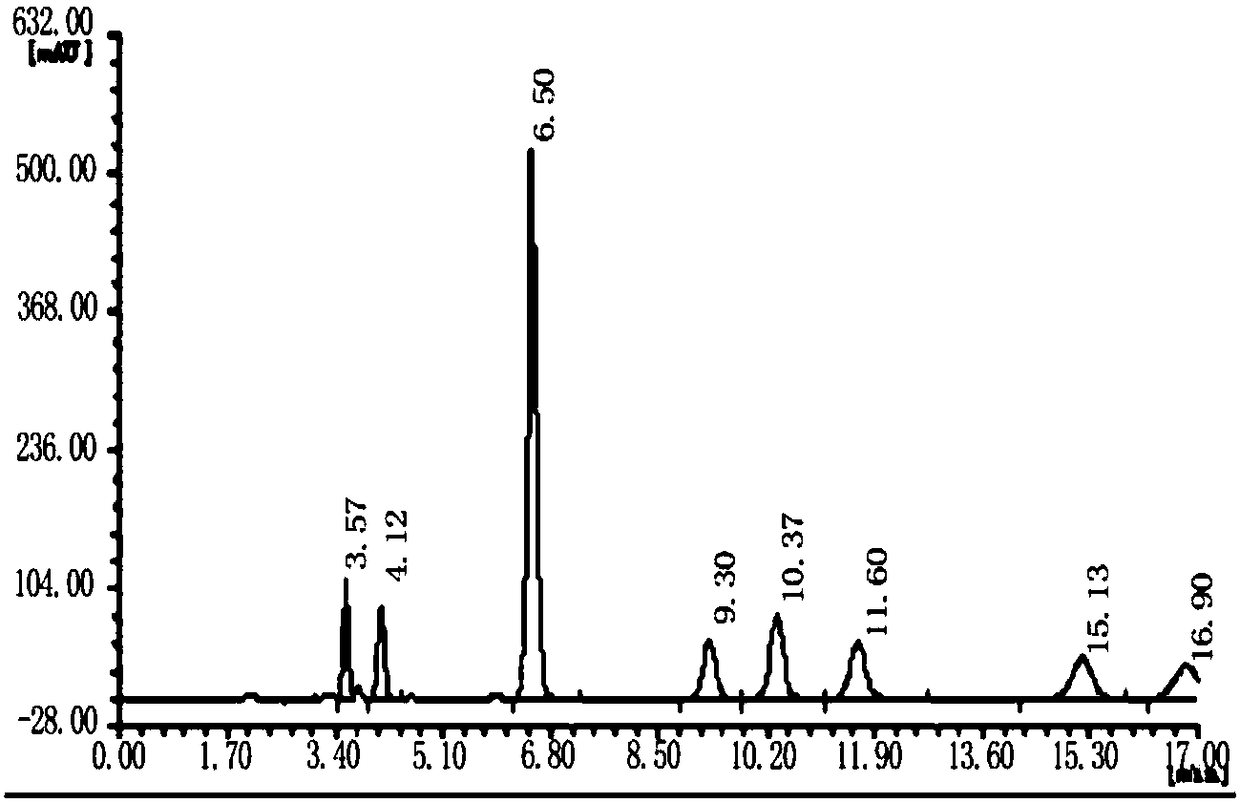
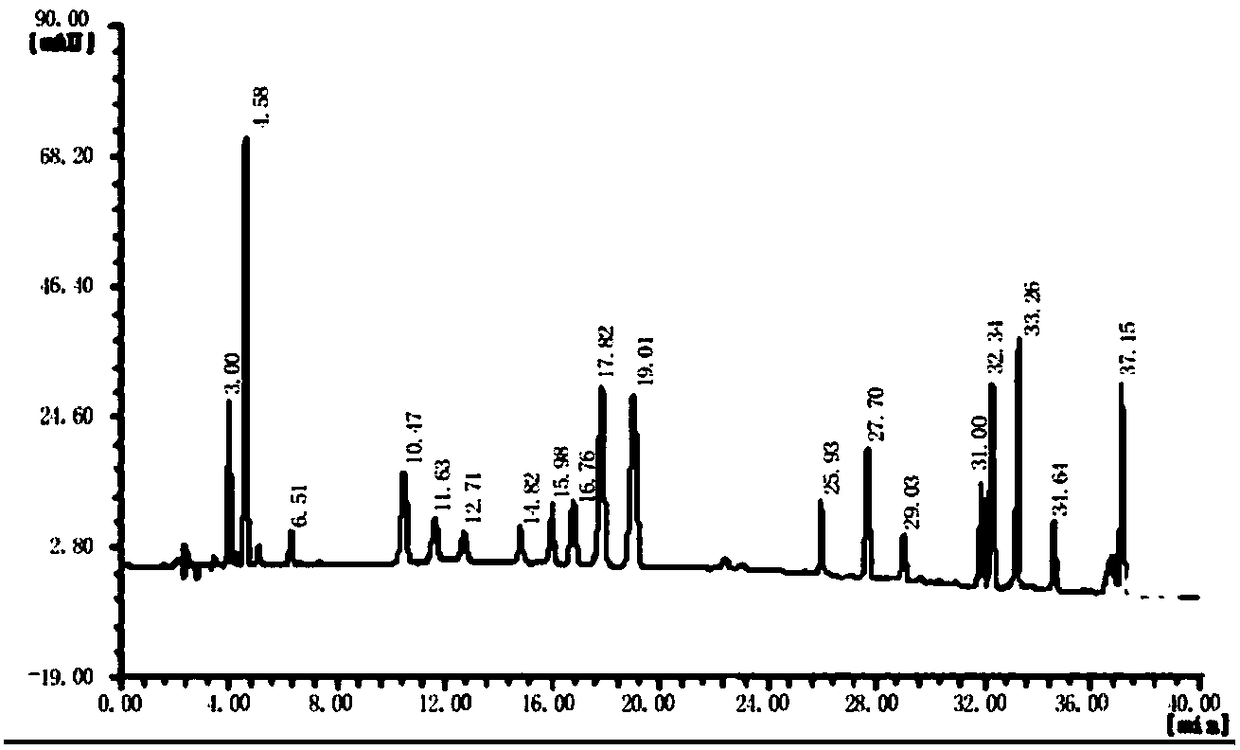
[0050] (1) Obtain collagen peptide: prepare collagen powder into a solution with a certain concentration, adjust the pH to 5.0-8.0, add an appropriate amount of protease hydrolysis solution, and react in a water bath at 40-60°C for 60-150min to obtain Different molecular weight collagen peptides. After the reaction is completed, inactivate in a boiling water bath, and store at low temperature for later use. Adjust the amount of enzyme added or reaction time as needed to produce collagen peptides with a molecular weight of 3000-5000 and a molecular weight of less than 1000. See the attached diagram for the molecular weight distribution. Figure 4 , 5 . Also commercially available, high molecular weight collagen peptide (high molecular weight fish collagen peptide GBB 50SP (molecular weight 2000-5000, purchased from Nitta Gelatin Co., Ltd., Japan)), low molecular weight collagen peptide (low...
Embodiment 2
[0058] Embodiment 2: the preparation method in the powder, granule, tablet, hard capsule, oral liquid and suspension of the composition of the present invention.
[0059] Powder: the ambient humidity is controlled below 40%, the temperature is 18-25°C, the raw and auxiliary materials are crushed, sieved, and weighed, and an appropriate amount of pharmaceutically acceptable auxiliary materials, the composition of Example 1, and other compounding materials are added to the mixer Raw materials and appropriate amount of pharmaceutically acceptable excipients, the mixing time is 5-30 minutes, the rotation speed is 20-30 r / min, the mixed powder is passed through a 20-40 mesh sieve, and quantitative packaging is sufficient. Moisture content <5%, and the microbial limit complies with relevant regulations.
[0060] Granules: Take the composition 8 of Example 1, and other compound raw materials and auxiliary materials in appropriate amounts. Mixing, spraying and other wet granulation, ...
Embodiment 3
[0065] Example 3: In the composition of the present invention, the composition of different ratios of high and low molecular weight collagen peptides and wheat peptides affects the body's absorption and metabolism rate.
[0066]Collagen peptide in the composition of this example: wheat peptide: casein hydrolyzate: gorse frondosa = 2.5: 2.2: 0.075: 0.
[0067] Rats were used to study the oral intake of different proportions of high and low molecular weight collagen peptides and wheat peptide compositions. Based on the time-varying curves of hydroxyproline and glutamine concentrations in plasma, the body’s response to high and low molecular weight collagen peptides was quantitatively analyzed. , The rate of absorption and metabolism of the active ingredients in wheat peptide, and optimize the optimal combination ratio. The characteristic substance in collagen peptide is hydroxyproline (Hyp), and the characteristic substance in wheat peptide is glutamine (Gln). A large number of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com