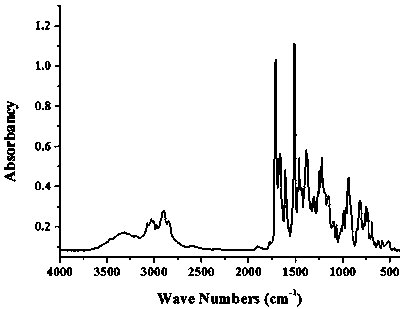
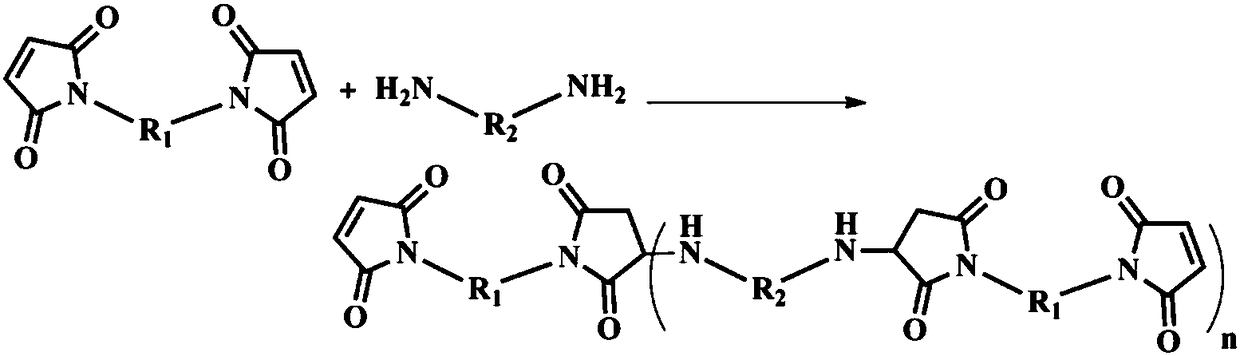
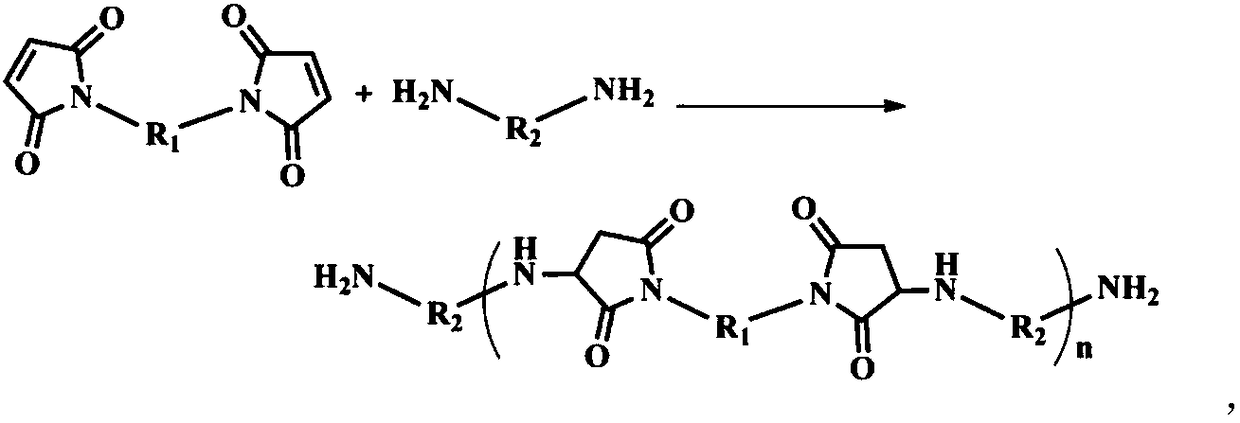
Method for modifying benzoxazine resin through chain extension in maleimide
A technology of maleimide and bismaleimide, which is applied in the field of thermosetting resin preparation, can solve the problems that the toughness of the polymer has not been significantly improved, the curing temperature of the benzoxazine resin is high, and the toughness of the cured product is poor. Achieve the effects of increasing molecular designability, promoting ring-opening reaction, and reducing polymerization reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 500ml of DMF into a three-necked flask equipped with a stirrer, a condenser, and a thermometer, slowly raise the temperature to 65°C, add 1mol (198g) of 4,4'-diaminodiphenylmethane, and pass in nitrogen protection. In this case, the diamine is completely dissolved. Then, slowly raise the temperature to 90°C, add 0.5mol (179g) of diphenylmethane type bismaleimide in batches within 30min, keep the temperature for 1.5h, and then slowly cool to 30-40°C to obtain a compound whose terminal group is an amino group. Bismaleimide and diamine chain extension product solution, kept warm for use.
[0044]Add 1mol (134g) of 2-allylphenol, 2.3mol (69g) of paraformaldehyde and 400ml of toluene into a four-neck flask equipped with a stirrer, condenser, thermometer, and constant pressure funnel, and slowly raise the temperature to 55-60 ℃. Gradually add the above-mentioned amino-terminated bismaleimide and diamine chain extension product solution to the system dropwise, and the dr...
Embodiment 2
[0047] Add 300ml of DMAc into a three-necked flask equipped with a stirrer, a condenser, and a thermometer, slowly heat up to 60°C, add 1.4mol (151.2g) of p-phenylenediamine, and pass in nitrogen protection. Under the condition of stirring, the two The amine is completely dissolved. Then, slowly raise the temperature to 90°C, add 0.5mol (179g) of diphenylmethane type bismaleimide in batches within 40min, keep the temperature for 1.0h, and then slowly cool to 30-40°C to obtain a compound whose terminal group is an amino group. Bismaleimide and diamine chain extension product solution, kept warm for use.
[0048] Add 1.8mol (169.2g) of phenol, 4.1mol (123g) of paraformaldehyde and 600ml of xylene into a four-neck flask equipped with a stirrer, condenser, thermometer, and constant pressure funnel, and slowly raise the temperature to 55-60°C. Gradually add the above-mentioned amino-terminated bismaleimide and diamine chain extension product solution to the system dropwise, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com