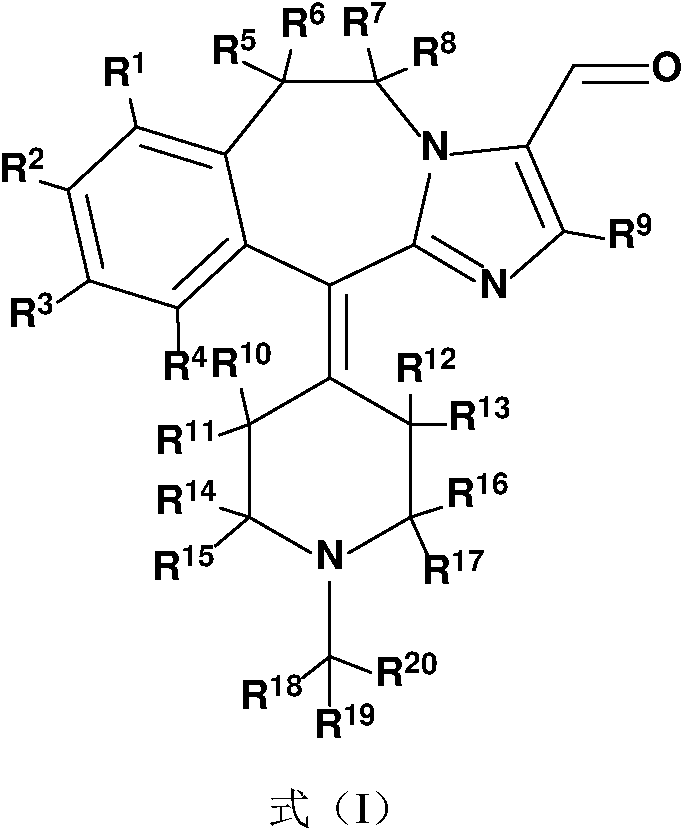
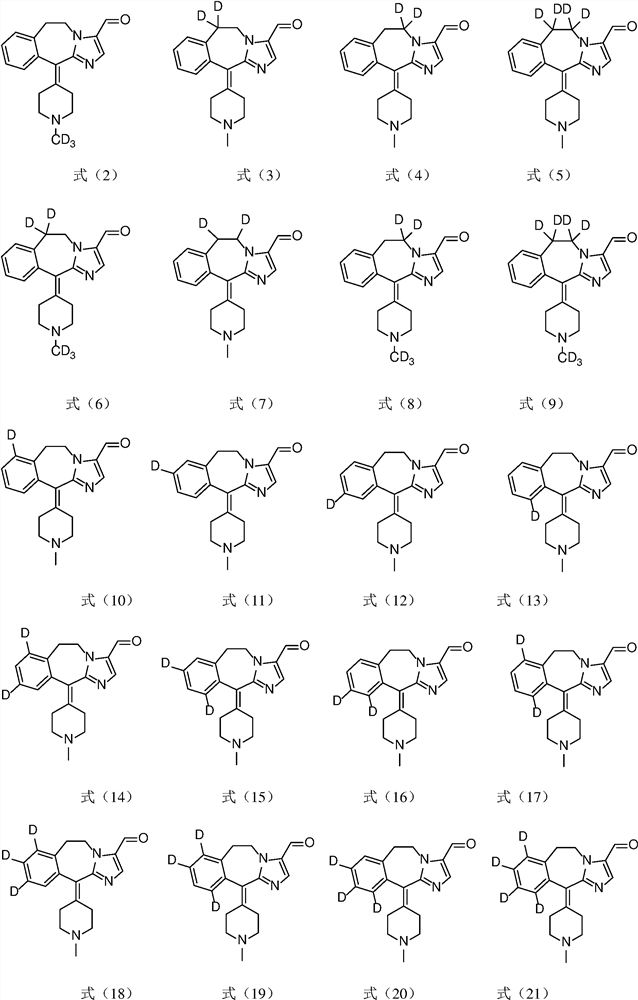
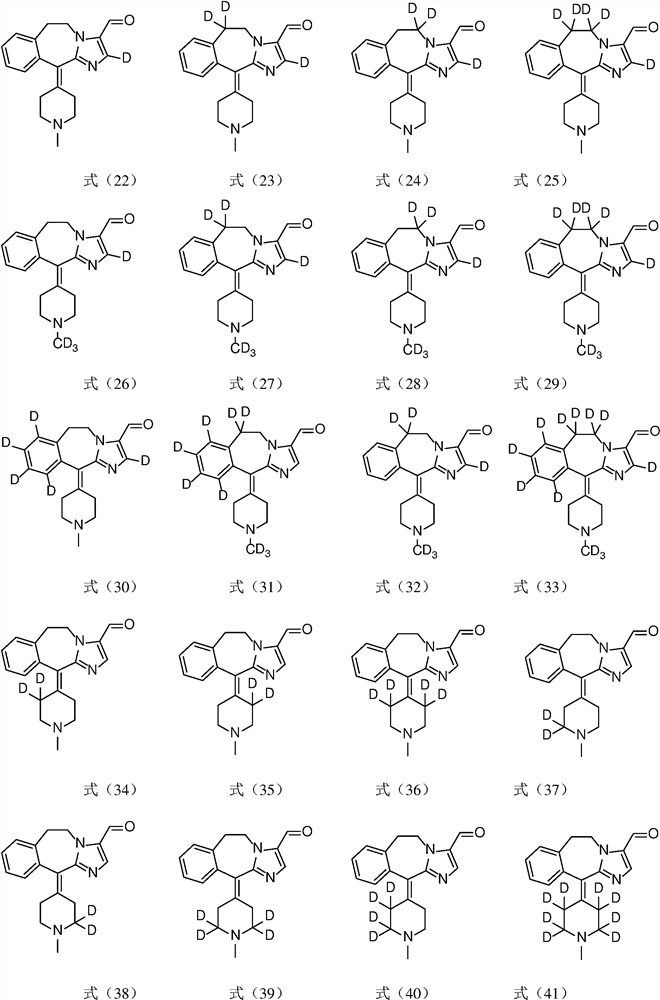
A substituted fused imidazole ring compound and its pharmaceutical composition
A compound and composition technology, applied in the field of medicine, can solve problems such as inability to alleviate eye allergy symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of 6,11-dihydro-11-(1-(d3-methyl)piperidin-4-ylidene)-5H-imidazo[2,1-b][3] Benzazepine -3-Formaldehyde (Compound 8)
[0050]
[0051] Concrete synthetic steps are as follows:
[0052]
[0053] Step 1. Synthesis of compound 3.
[0054] Dissolve N-benzyloxycarbonylpiperidine-4-carboxylic acid (2.63 g, 10 mmol) in 20 mL of dichloromethane, add 6 mL of oxalyl chloride and 1 drop of DMF, and react at room temperature for 2 hours under nitrogen protection. Concentrate the reaction solution to dryness under reduced pressure, add 20 mL of acetonitrile to dissolve, add triethylamine (4.1 mL, 30 mmol) under ice-cooling, and stir for 3 minutes. A solution of 1-phenethyl-1H-imidazole (2.06 g, 12 mmol) in 5 mL of acetonitrile was slowly added dropwise, and after the addition was completed, the reaction was allowed to rise to room temperature overnight. After completion of the reaction, concentrate to dryness, add 30mL ethyl acetate and 20mL water...
Embodiment 2
[0065] Example 2 Preparation of 6,6-d2-6,11-dihydro-11-(1-methylpiperidin-4-ylidene)-5H-imidazo[2,1-b] [3] Benzazepines -3-Formaldehyde (compound 19)
[0066]
[0067] Concrete synthetic steps are as follows:
[0068]
[0069] Step 1. Synthesis of compound 10.
[0070] Phenylacetic acid (3.15g, 23mmol) was added to 10mL of 3.5M heavy aqueous solution of deuterated sodium oxide, reacted at 100°C for 24 hours under nitrogen protection, cooled to room temperature, the reaction solution was acidified with 4N hydrochloric acid, extracted with dichloromethane, and the organic phase was After drying with anhydrous sodium sulfate and concentrating, the above operation was repeated once more for the obtained crude product, and finally about 2.88 g of compound 10 was obtained by silica gel column separation, with a yield of 90%. 1 H NMR (400MHz, DMSO-d 6 )δ12.30(s, 1H), 7.34-7.21(m, 5H); ESI-MS: 139[M + +1].
[0071] Step 2. Synthesis of Compound 11.
[0072] Lithium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com