Tacrolimus nanocrystal and artificial tear compound and preparation method thereof
A technology of tacrolimus and artificial tears, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of low drug efficacy, poor stability and bioavailability, and low patient compliance , to achieve good reproducibility, good application prospects, and reduce the effect of direct stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of tacrolimus nanocrystal
[0044] The preparation method of tacrolimus nanocrystal specifically comprises the following steps:
[0045] Mix the tacrolimus drug crystals with the absolute ethanol solution so that the concentration is 4 mg / mL to obtain the tacrolimus absolute ethanol solution. The above solution was added to the aqueous solution at a dropping rate of 2 mL / min (the volume ratio of tacrolimus absolute ethanol solution to aqueous solution was 1:10), and stirred at a high speed of 7200 rpm to obtain a suspension. The suspension was continuously stirred at a rate of 800 rpm for 12 h, and absolute ethanol in the solution was volatilized to obtain a final solution. The final solution was centrifuged several times to remove the supernatant to obtain tacrolimus nanocrystals.
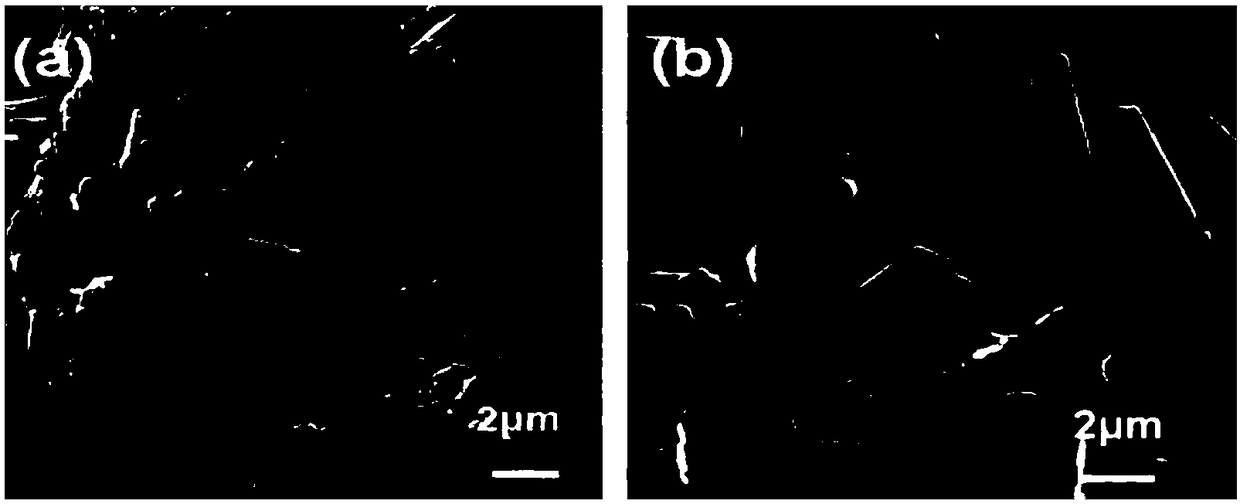
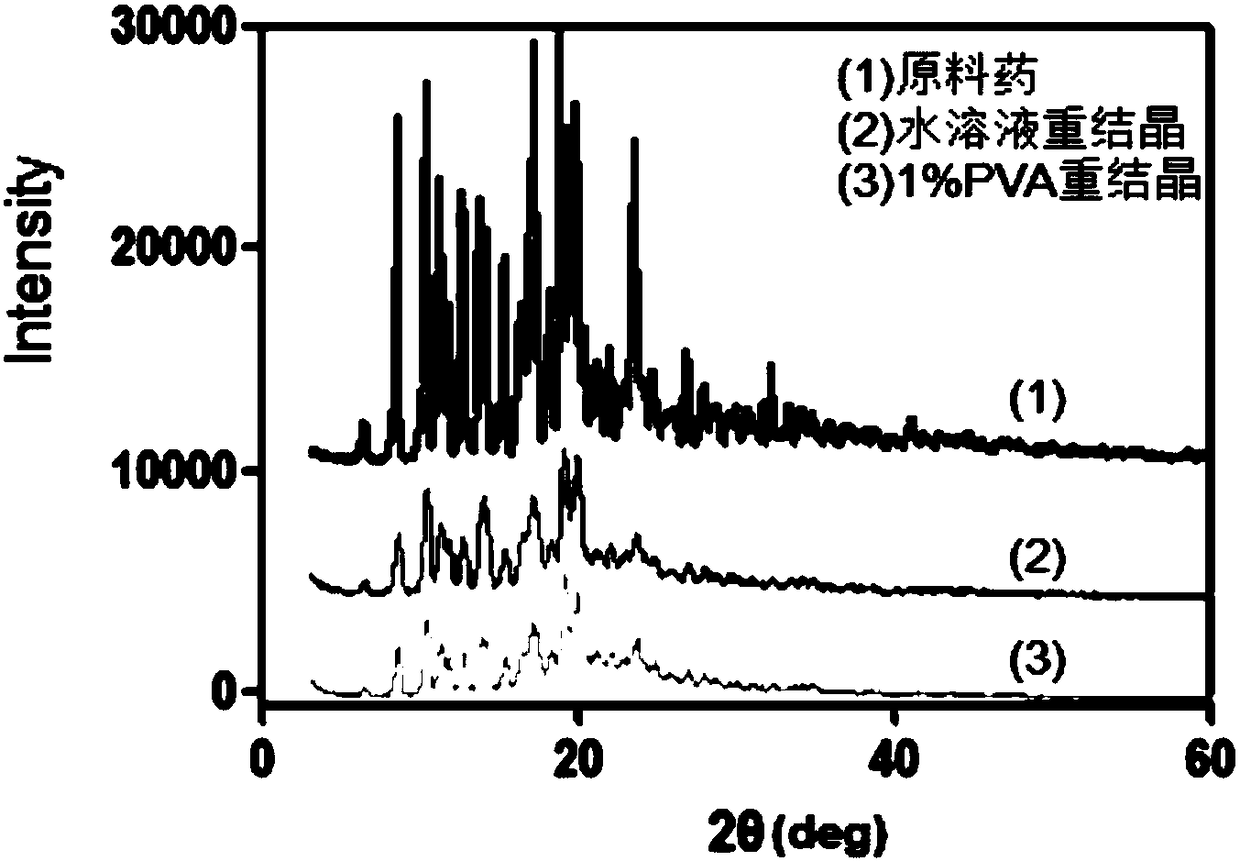
[0046] Its electron microscope picture is as figure 1 As shown in (a), by figure 1 (a) It can be seen that the crystal form of the tacrolimus nanocrystal i...
Embodiment 2
[0047] Embodiment 2, the preparation of tacrolimus nanocrystal
[0048] The preparation method of tacrolimus nanocrystal specifically comprises the following steps:
[0049] Mix the tacrolimus drug crystals with the absolute ethanol solution so that the concentration is 4 mg / mL to obtain the tacrolimus absolute ethanol solution. Add the above solution to a polyvinyl alcohol (PVC for short) aqueous solution with a mass volume concentration of 1 g / L at a dropping rate of 2 mL / min (the volume ratio of tacrolimus absolute ethanol solution to PVC aqueous solution is 1:10) , stirring at a high speed of 7200rpm to obtain a suspension. The suspension was continuously stirred at a rate of 800 rpm for 12 h, and absolute ethanol in the solution was volatilized to obtain a final solution. The final solution was centrifuged several times to remove the supernatant to obtain tacrolimus nanocrystals.
[0050] Its electron microscope picture is as figure 1 (b), shown by figure 1 (b) It ca...
Embodiment 3
[0051] Embodiment 3, the preparation of tacrolimus nanocrystal
[0052] The preparation method of tacrolimus nanocrystal specifically comprises the following steps:
[0053] Mix the tacrolimus drug crystals with the absolute ethanol solution so that the concentration is 4 mg / mL to obtain the tacrolimus absolute ethanol solution. The above solution was added to a polyvinyl alcohol aqueous solution with a mass volume concentration of 1 g / L (the volume ratio of tacrolimus absolute ethanol solution to PVC aqueous solution was 1:10) at a dropping rate of 2 mL / min, and was added at a rate of 11200 rpm. Stir at high speed to obtain a suspension. The suspension was continuously stirred at a rate of 800 rpm for 12 h, and absolute ethanol in the solution was volatilized to obtain a final solution. The final solution was centrifuged several times to remove the supernatant to obtain tacrolimus nanocrystals.
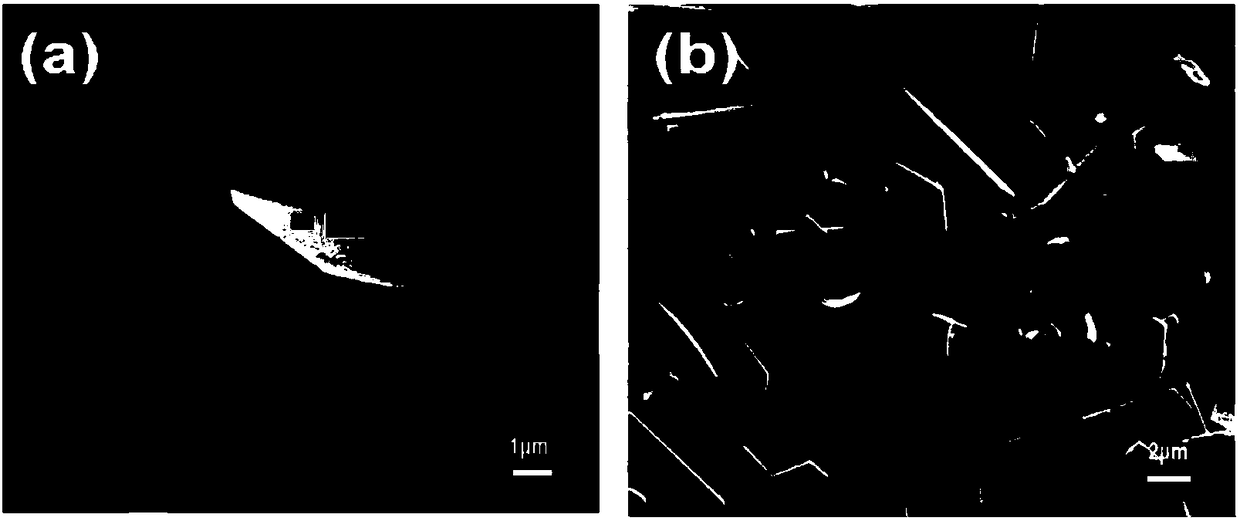
[0054] Its electron microscope picture is as figure 2 As shown in (a), by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com