Extracellular vesicle-containing injectable hydrogel for treating erectile dysfunction and preparation method of extracellular vesicle-containing injectable hydrogel
A technology for erectile dysfunction and water injection, which is applied in the field of biomedicine, can solve problems such as poor effect and large side effects, and achieve the effects of promoting vascular endothelial repair, nerve regeneration, and recovery of erectile function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A medicine for treating erectile dysfunction and a preparation method thereof, comprising the steps of:
[0029] (1) Human mesenchymal stem cell-derived extracellular vesicle extraction: P4 adipose-derived mesenchymal stem cells obtained from the patient's own tissue or cell bank were cultured in clinical-grade human mesenchymal stem cell serum-free medium. When the cell fusion reached 80- At 90%, serum-free DMEM medium was added, and the cell supernatant was collected 24 h later. The cell supernatant was centrifuged at 300 g for 10 min at 4°C, 16,500 g for 10 min at 4°C to remove cells and cell debris, and 120,000 g for 70 min at 4°C to collect extracellular vesicles.
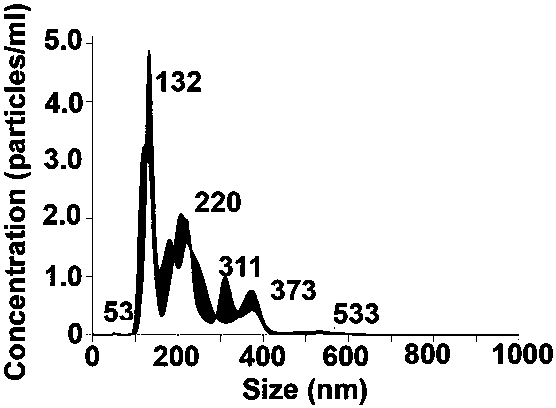
[0030] (2) Quantification of extracellular vesicles derived from human mesenchymal stem cells: The extracellular vesicles derived from human mesenchymal stem cells were diluted 1000 times in phosphate buffer, according to the Brownian motion and diffusion coefficient in the Nanosight instrument (NS300, ...
Embodiment 2
[0037] Example 2 Treatment of Diabetic Erectile Dysfunction in Sprague-Dawley Rats.
[0038] (1) Extracellular vesicles derived from P4 human adipose stem cells were prepared according to the above steps in Example 1 and mixed with p-carboxybenzaldehyde-modified polyethylene glycol-hydroxyethyl chitosan hydrogel to prepare encapsulating human adipose stem cells Injectable hydrogel formulation of p-carboxybenzaldehyde-modified polyethylene glycol-hydroxyethyl chitosan derived from extracellular vesicles.
[0039] (2) A diabetic erectile dysfunction model was established on 8-week-old Sprague-Dawley rats, and 100 μm of the above-mentioned injectable hydrogel preparation encapsulated with human mesenchymal stem cells was injected into the rat corpus cavernosum.
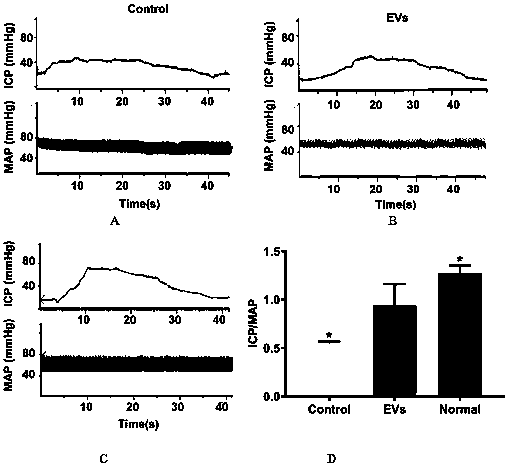
[0040] (3) After 2 weeks of treatment, evaluate the erectile function of the rats, measure the intracavernous pressure (ICP) and mean arterial pressure (MAP), and calculate the ratio of the two.
[0041] Depend on ima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com