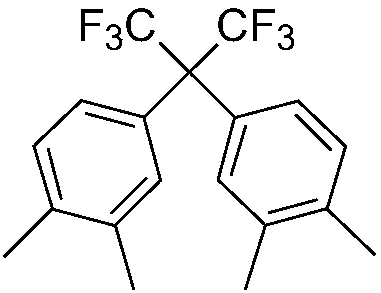
Preparation method of 2,2-bis(3,4-dimethylphenyl) hexafluoropropane
A technology of hexafluoropropane and xylyl, which is applied in 2, can solve the problems of difficulty in obtaining ionic liquids, high reaction pressure, and complicated equipment, and achieve great implementation value and social and economic benefits, mild preparation conditions, and low risk factors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] In some embodiments, the preparation method includes at least the following steps:
[0037] 1), mixing o-xylene, sulfonic acid and solvent in proportion to obtain a mixture;
[0038]2), pass hexafluoroacetone gas into the mixture in step 1), and react under the reaction temperature and reaction pressure to obtain a crude product, wash the crude product with alkali, wash with water, concentrate, crystallize through a crystallization solvent, and dry in vacuo to obtain 2,2-bis(3,4-xylyl)hexafluoropropane.
[0039] In some embodiments, the molar ratio of o-xylene to sulfonic acid is 1.0:0.1˜1.0:1.0. ,
[0040] In some embodiments, the sulfonic acid has the general formula C x h y f z SO 3 H, X is a number from 1 to n, Y is a number from 1 to n, and Z is a number from 0 to n.
[0041] In some embodiments, the sulfonic acid is selected from methanesulfonic acid, trifluoromethanesulfonic acid, 1-trifluoroethylsulfonic acid, pentafluoroethylsulfonic acid, propylsulfonic...
Embodiment 1
[0052] Example 1: In a 1000 ml glass bottle, add 600 g of 1,2-dichloroethane, 212 g of o-xylene, 15 g of trifluoromethanesulfonic acid, control the temperature at 30°C, and slowly feed 250 g of hexafluoroacetone After the gas is passed through, the temperature is raised to reflux for 6 hours, the temperature is lowered, and NaHCO is used to 3 Saturated aqueous solution, washed to pH = 7 ~ 8, the organic phase was washed once with pure water, concentrated, the obtained solid was recrystallized with 600 g of isopropanol, and the wet product was vacuum dried at 40 ° C for 12 hours to obtain 330 g of the product. The yield 91.7%, GC purity 99.5%, single impurity less than 0.1%, all metal ions less than 1ppm.
Embodiment 2
[0053] Example 2: In a 2000 ml glass bottle, add 1000 grams of tetrahydrofuran, 424 grams of o-xylene, 60 grams of p-toluenesulfonic acid, control the temperature below 40°C, and slowly feed 400 grams of hexafluoroacetone gas. , heat up to reflux for 12 hours, cool down, use NaHCO 3 Saturated aqueous solution, washed to pH = 7-8, added 500 grams of toluene, layered, the organic phase was washed once with pure water, concentrated, the obtained solid was recrystallized with 1200 grams of ethanol, and the wet product was vacuum-dried at 40°C for 12 hours to obtain The product was 670 grams, the yield was 93.1%, the GC purity was 99.6%, individual impurities were less than 0.1%, and all metal ions were less than 1ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com