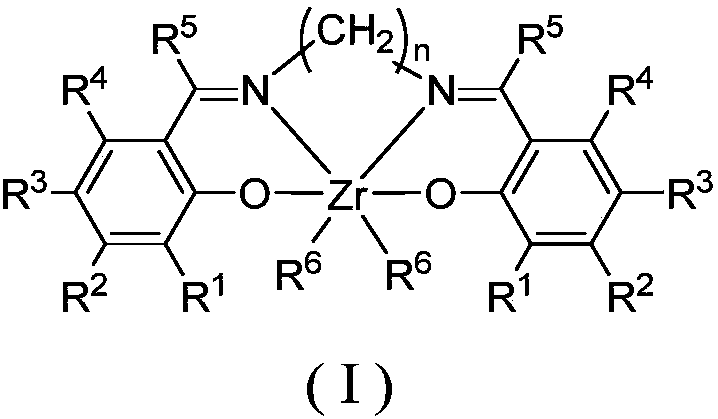
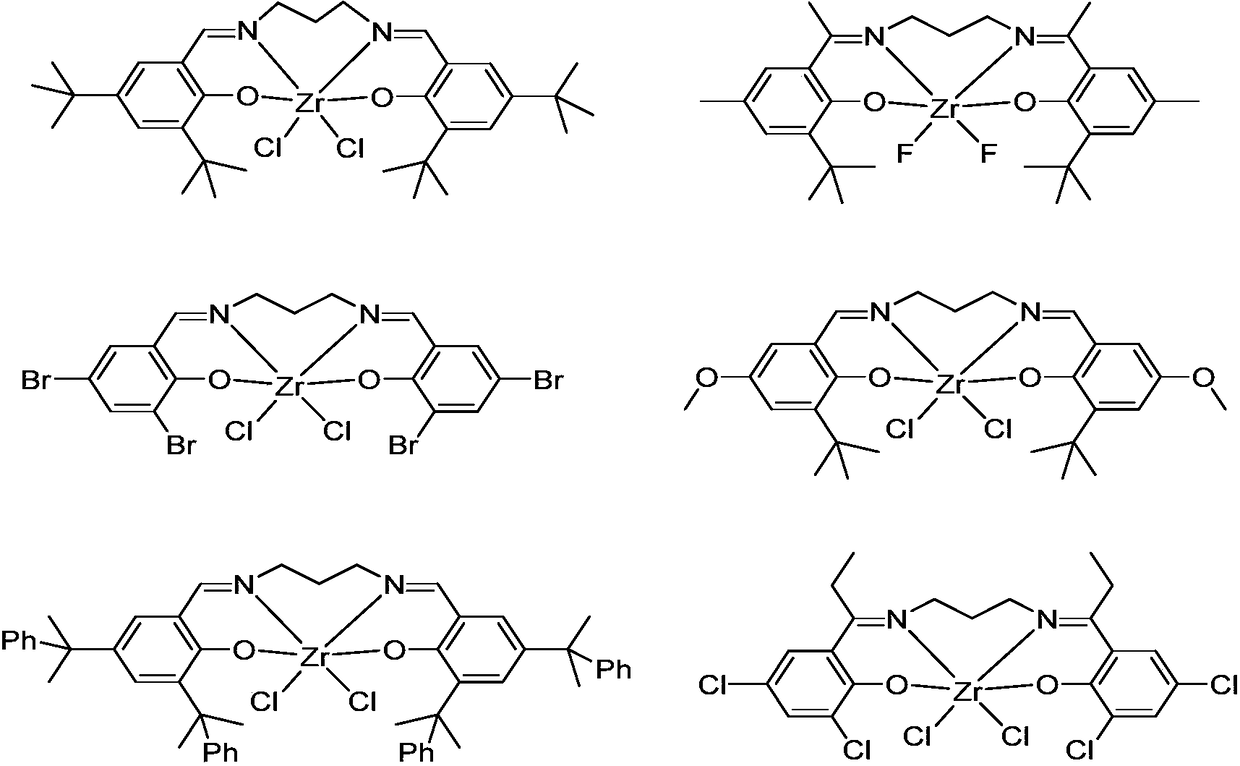
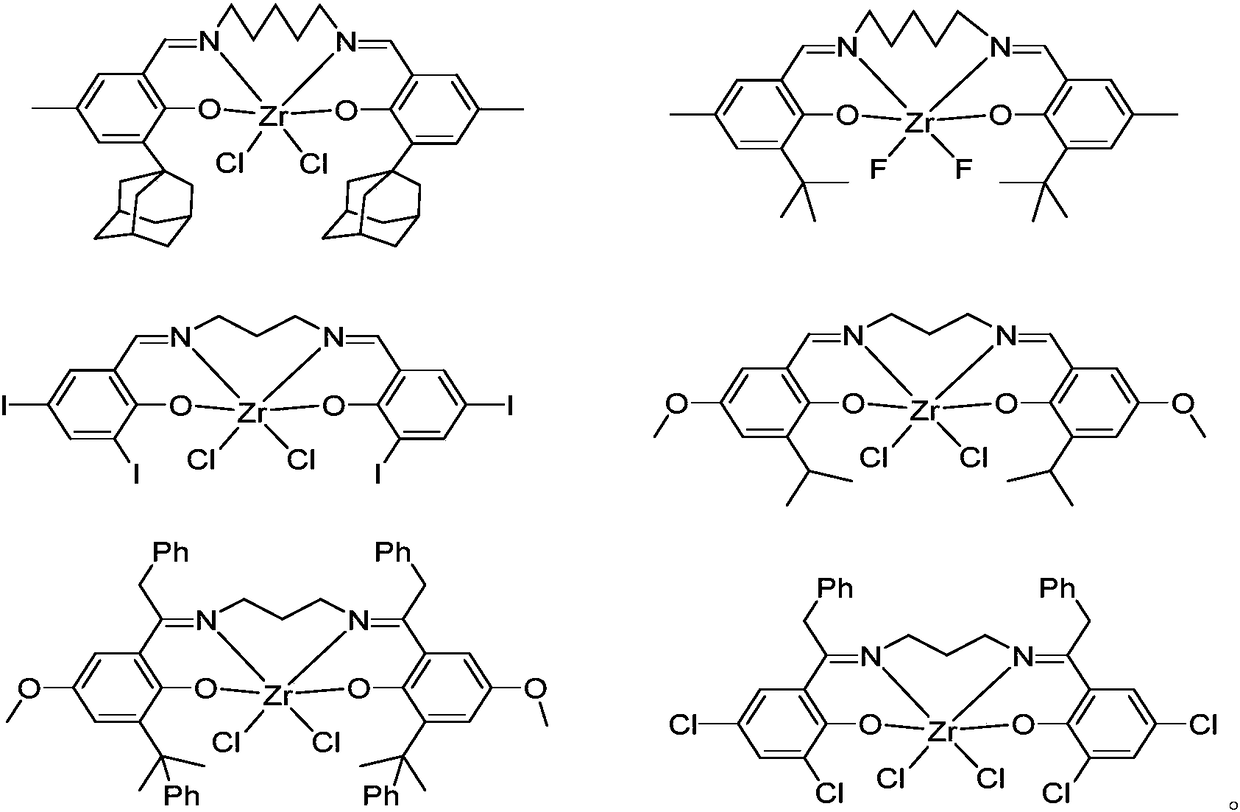
Bisphenol oxygen-imine ligand zirconium compound, as well as preparation method and application thereof
A technology of bisphenoloxyimine and zirconium compound, applied in the field of olefin polymerization, can solve the problems of limited application scope, high price, and many catalyst synthesis steps, and achieves the effects of overcoming low molecular weight, high catalytic activity and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of Ligand L1
[0035] Add 3,5-di-tert-butyl salicylaldehyde (4.68g, 20mmol), absolute ethanol (50mL) and 1.0g molecular sieves to the 100mL reaction flask successively Stir at room temperature for 5 minutes, add 1,3-propanediamine (0.74 g, 10 mmol), and react under reflux for 16 hours. The reaction liquid was filtered, and the solvent was removed from the filtrate under reduced pressure, and the product (4.11 g, yield: 81.1%) was obtained by recrystallization from petroleum ether.
[0036]
[0037] 1 H NMR (CDCl 3 ,400MHz):δ10.93(s,2H,OH),8.56(s,2H,CH=N),7.20(d,2H, 4 J=2.2Hz, ArH), 6.80(d, 2H, 4 J=2.2Hz, ArH), 3.71(t, 4H, 3 J=7.5Hz, NCH 2 ),2.01(m,2H,CH 2 CH 2 CH 2 ),1.43(s,18H,C(CH 3 ) 3 ),1.28(s,18H,C(CH 3 ) 3 ). 13 C NMR (CDCl 3 ,100MHz): δ161.4,154.3,140.4,135.4,123.2,122.7,121.5,59.4,34.5,32.2,31.6,25.0.Anal.Calcd.forC 33 h 50 N 2 o 2 : C, 78.21; H, 9.95; N, 5.53. Found: C, 78.47; H, 10.02; N, 5.77%.
Embodiment 2
[0039] Synthesis of Ligand L2
[0040] Add 3-tert-butyl-5-methyl salicylaldehyde (3.84g, 20mmol), absolute ethanol (50mL) and 1.0g molecular sieves to the 100mL reaction flask successively Stir at room temperature for 5 minutes, add 1,3-propanediamine (0.74 g, 10 mmol), and react under reflux for 16 hours. The reaction liquid was filtered, and the solvent was removed from the filtrate under reduced pressure, and the product (2.87 g, yield: 70.2%) was obtained by recrystallization from petroleum ether.
[0041]
[0042] 1 H NMR (CDCl 3 ,400MHz):δ10.79(s,2H,OH),8.44(s,2H,CH=N),7.37(d,2H, 4 J=2.4Hz, ArH), 7.08(d, 2H, 4 J=2.4Hz, ArH), 3.95(t, 4H, 3 J=7.2Hz, NCH 2 ),2.36(s,6H,ArCH 3 ),1.40(s,18H,C(CH 3 ) 3 ). 13 C NMR (CDCl 3 ,100MHz): δ161.4,157.3,140.4,135.4,123.2,122.7,121.5,61.9,34.5,31.6,21.6.Anal.Calcd.for C 26 h 36 N 2 o 2 : C, 76.43; H, 8.88; N, 6.86. Found: C, 76.47; H, 9.02; N, 6.77%.
Embodiment 3
[0044] Synthesis of Ligand L3
[0045] Add 2-hydroxy-3-cumyl-5-methoxybenzophenone (5.69g, 20mmol), absolute ethanol (50mL), 1 drop of formic acid and 1.0g molecular sieves to the 100mL reaction flask successively Stir at room temperature for 5 minutes, add 1,3-propanediamine (0.74 g, 10 mmol), and react under reflux for 16 hours. The reaction liquid was filtered, and the solvent was removed from the filtrate under reduced pressure, and the product was obtained by recrystallization from petroleum ether (3.25 g, yield: 53.6%).
[0046]
[0047] 1 H NMR (CDCl 3 ,400MHz):δ10.12(s,2H,OH),7.35-7.13(m,12H,ArH),6.85(d,2H, 4 J=2.4Hz, ArH), 3.81(s, 6H, OCH 3 ),3.67(t, 3 J=7.2Hz, 4H, NCH 2 ),2.11(m,2H,CH 2 CH 2 CH 2 ),1.81(s,6H,NCCH 3 ),1.69(s,12H,CPh(CH 3 ) 2 ). 13 CNMR (CDCl 3 for C 39 h 46 N 2 o 4 : C, 77.20; H, 7.64; N, 4.62. Found: C, 77.41; H, 7.75; N, 4.56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com