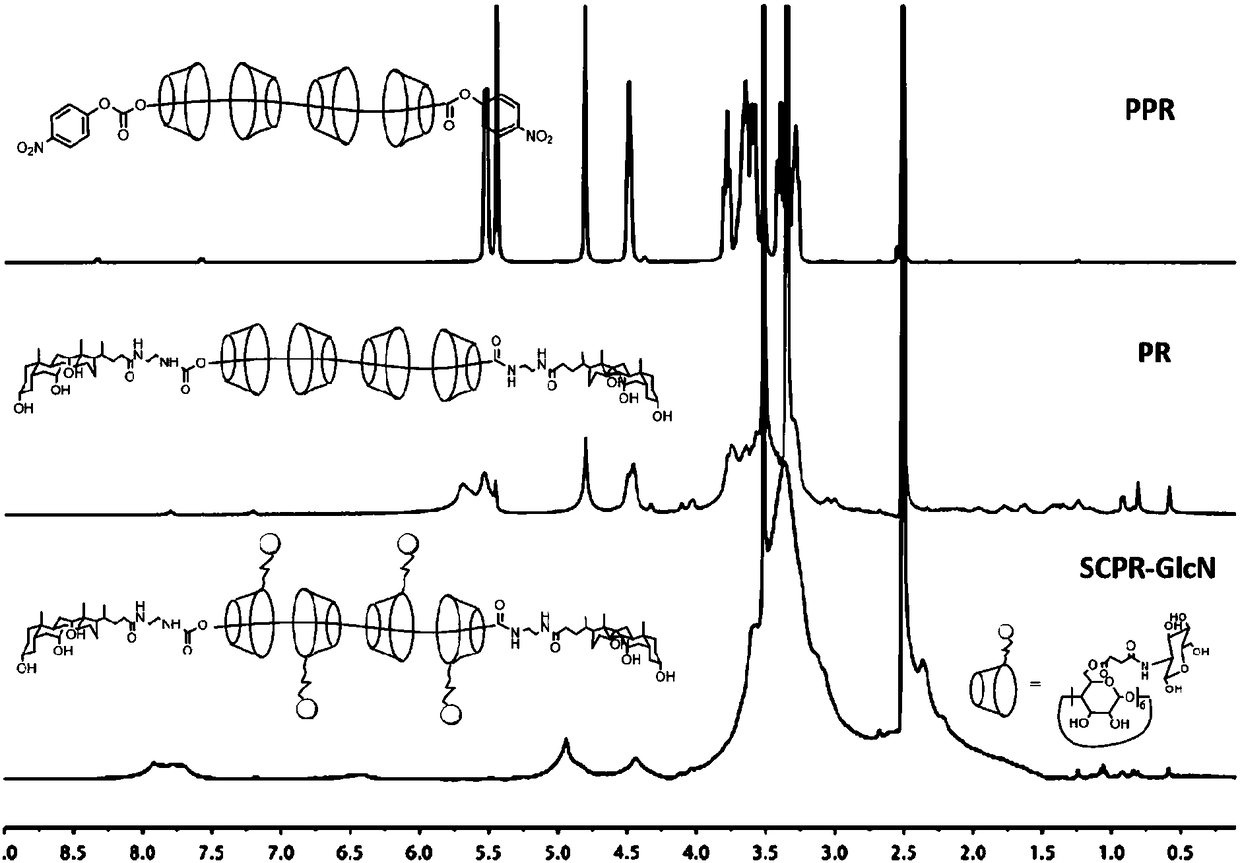
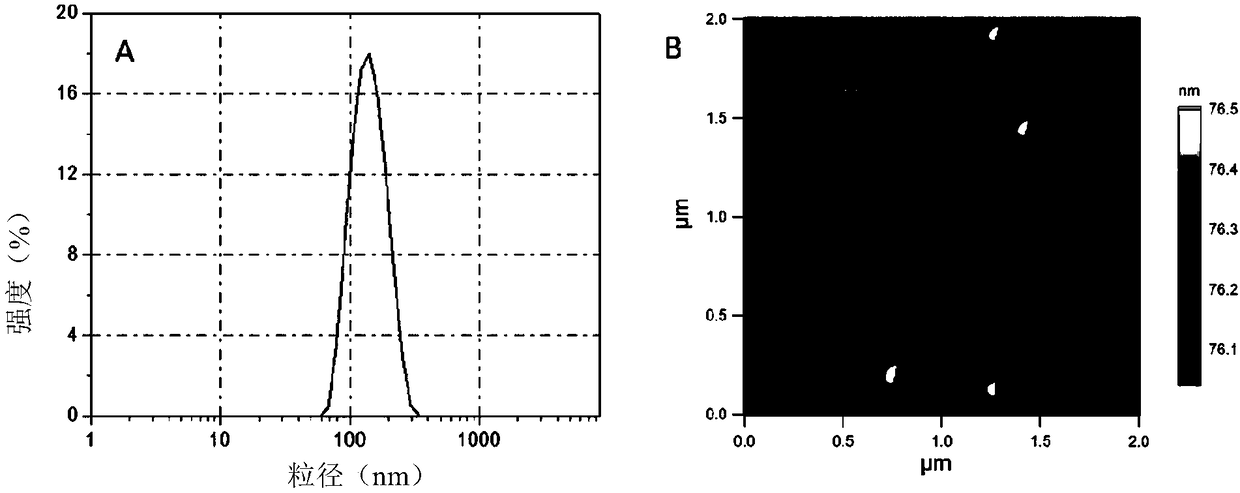
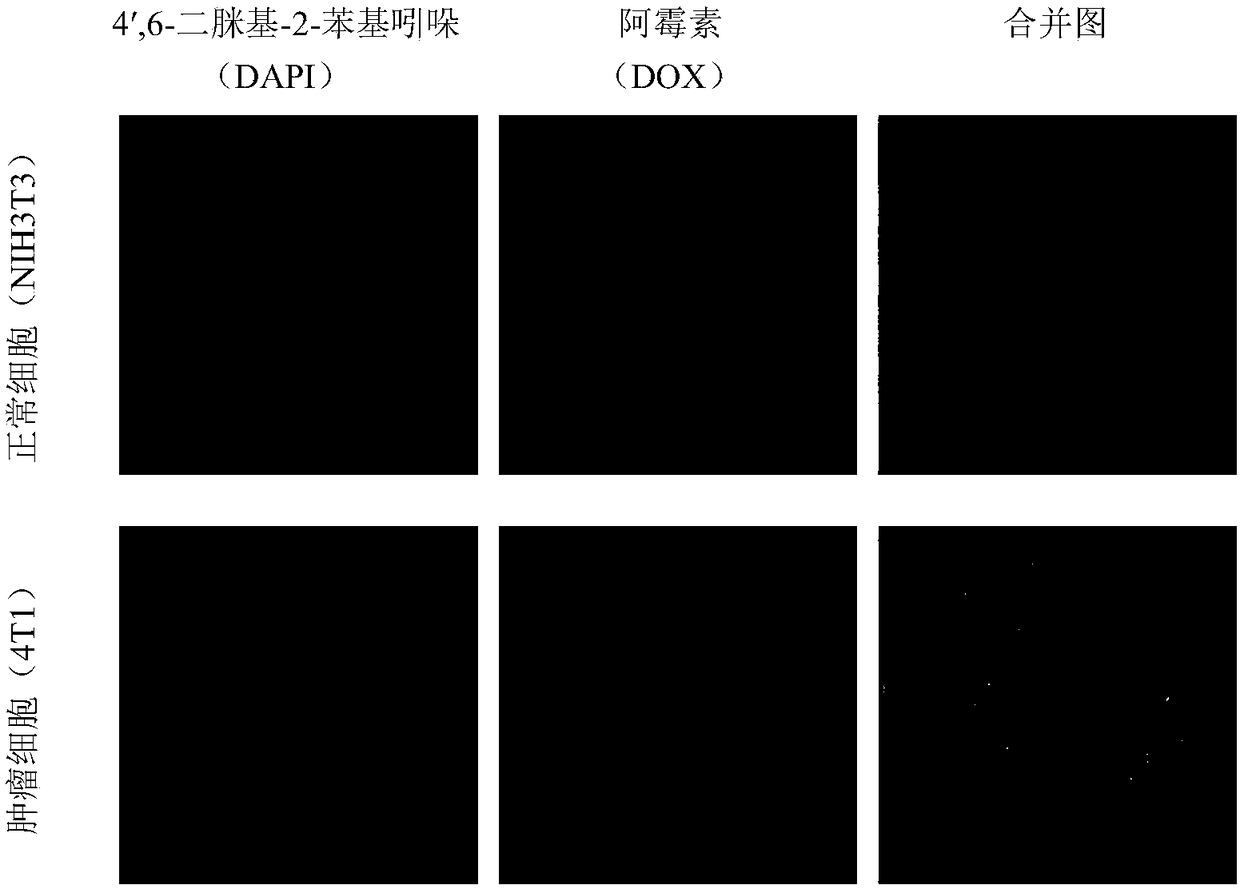
Modified polyrotaxane, medicine carrier micelle based on polyrotaxane and preparation method and application of micelle
A technology of drug-loaded micelles and polyrotaxanes, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, antineoplastic drugs, etc., can solve the problem of low encapsulation efficiency of drugs, achieve clear hydrophilic and hydrophobic structures, and improve encapsulation. terminal efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) Preparation of pseudopolyrotaxane: Dissolve activated ester modified polyethylene glycol (1g, 0.43mmol) (molecular weight: 2330, molar mass of activated ester unit: 0.86mmol) in 5mL deionized water, and add Added to a saturated aqueous solution of α-cyclodextrin (8.35g, 8.56mmol), a white precipitate formed after a few minutes, sonicated for 1.5h (ultrasonic frequency: 40kHz, power: 100W), stirred at room temperature (speed: 400rpm) for 12h, and collected by centrifugation Precipitate, freeze-dried to obtain quasi-polyrotaxane PPR;
[0065] (2) Preparation of polyrotaxane: Dissolve amino-modified cholic acid (0.32g, 0.71mmol) (molecular weight: 450) in 4mL DMF, and add dropwise to pseudopolyrotaxane PPR (4g, 0.236mmol, activated ester unit The molar weight is 0.47mmol) powder, stir until a yellow viscous liquid is formed, add triethylamine (10μL, 0.072mmol) dropwise to catalyze, ultrasonically treat for 1.5h (ultrasonic frequency: 40kHz, power: 100W), and react at 5...
Embodiment 2
[0074] (1) Preparation of pseudopolyrotaxanes: Dissolve activated ester modified polyethylene glycol (1g, 0.43mmol) in 5mL deionized water, and add dropwise to α-cyclodextrin (7.52g, 7.73mmol) In the saturated solution, a white precipitate formed after a few minutes, sonicated for 1h (ultrasonic frequency: 40kHz, power: 200W), stirred at room temperature (speed: 400rpm) for 18h, centrifuged to collect the precipitate, and freeze-dried to obtain PPR;
[0075] (2) Preparation of polyrotaxane: Dissolve amino-modified cholic acid (0.43g, 0.96mmol) in 4mL DMF, and add dropwise to pseudopolyrotaxane PPR (4g, 0.27mmol, molar mass of activated ester unit is 0.54 mmol) powder, stirred until a yellow viscous solution was formed, triethylamine (8 μL, 0.054 mmol) was added dropwise to catalyze, ultrasonic treatment was performed for 1 h (ultrasonic frequency: 40 kHz, power: 200 W), reaction was carried out at 40°C for 36 h, and ether precipitated. The precipitate was dissolved in a small ...
Embodiment 3
[0080] (1) Preparation of pseudopolyrotaxanes: Dissolve activated ester-modified polyethylene glycol (1 g, 0.43 mmol) in 5 mL of deionized water, and add dropwise to α-cyclodextrin (10.44 g, 10.73 mmol) In the saturated solution, a white precipitate formed after a few minutes, sonicated for 2h (ultrasonic frequency: 40kHz, power: 100W), stirred at room temperature (speed: 300rpm) for 24h, centrifuged to collect the precipitate, and freeze-dried to obtain PPR;
[0081] (2) Preparation of polyrotaxane: Dissolve amino-modified cholic acid (0.41g, 0.9mmol) in 4mL DMF, and add dropwise to pseudopolyrotaxane PPR (4g, 0.18mmol, molar mass of activated ester unit is 0.36mmol ) powder, stirred until a yellow viscous solution was formed, triethylamine (8 μL, 0.054 mmol) was added dropwise to catalyze, ultrasonic treatment was performed for 2 h (ultrasonic frequency: 40 kHz, power: 100 W), reaction was carried out at 50 °C for 24 h, and ether precipitated. The precipitate was dissolved i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com