Highly-hydrophilic adsorbent as well as preparation and application to absorption of rubidium ions or lithium ions
An adsorbent and hydrophilic technology, applied in the field of adsorbent preparation, can solve problems such as dissolution and instability, and achieve the effects of fast analysis rate, improved production efficiency, and fast adsorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of a kind of highly hydrophilic adsorbent of the present invention, first, powdery lithium or rubidium ion adsorbent is added in the aqueous solution of hydrophilic polymer, fully stirs and mixes and drips into the phase inversion agent in the form of droplet In this method, the nascent adsorbent in the form of a sphere is formed by solution phase inversion; then, the nascent spherical adsorbent is added to the toluene-2,4-diisocyanate solution with n-hexane as the solvent for chemical cross-linking reaction , to obtain a highly hydrophilic adsorbent.
[0028] The highly hydrophilic adsorbent prepared by the present invention is mainly used for absorbing and extracting rubidium ions or lithium ions from seawater, brine, lepidolite acid leaching lithium raffinate, lithium carbonate precipitation and lithium raffinate, etc.
Embodiment 1
[0031] Carboxymethyl cellulose was prepared into 10 mL of an aqueous solution with a mass concentration of 6%, and then 0.6 g of manganese-based lithium ion sieve adsorbent was added; stirred evenly to form a viscous state, manually pressed out with a syringe and dropped into the phase inversion agent tetrahydrofuran solution , after 3 minutes of phase inversion reaction, it becomes a spherical solid particle, and the nascent lithium ion spherical adsorbent is obtained.
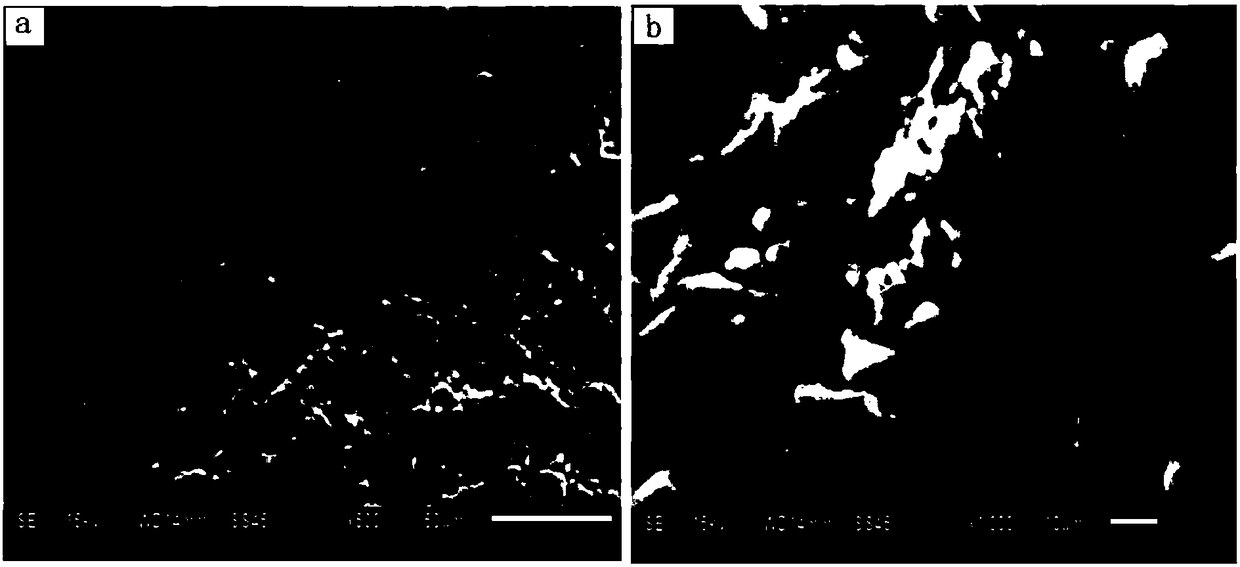
[0032] Take out the nascent spherical adsorbent and dry it at 60°C, place it in a n-hexane solution of toluene-2,4-diisocyanate with a concentration of 1% by mass and volume at 30°C, and take it out after reacting for 30 minutes. A spherical highly hydrophilic lithium ion adsorbent is obtained. The morphology of the adsorbent is as figure 1 said, figure 1 (a) is a 500-fold magnified topography of the adsorbent surface, figure 1 (b) is the 1000 times magnified topography image of the adsorbent surface.
[...
Embodiment 2
[0035] Take 4% aqueous solution of hydroxyethyl cellulose, take 10mL and add 0.48g of manganese-based lithium ion sieve adsorbent to it, mix and stir evenly to become viscous, manually press out with a syringe and drop into the phase inversion agent ether solution , after 3 minutes of phase inversion reaction, it becomes a spherical solid particle, and the nascent lithium ion spherical adsorbent is obtained.
[0036] Place the nascent spherical adsorbent at 30°C in a n-hexane solution of toluene-2,4-diisocyanate with a mass concentration of 1.5%, take it out after reacting for 30 minutes, and obtain a spherical highly hydrophilic lithium ion adsorbent . The morphology of the adsorbent is as figure 2 said, figure 2 (a) is a 500-fold magnified topography of the adsorbent surface, figure 2 (b) is the 1000 times magnified topography image of the adsorbent surface.
[0037] The prepared spherical lithium ion adsorbent is loaded into the adsorption column for the preparation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com