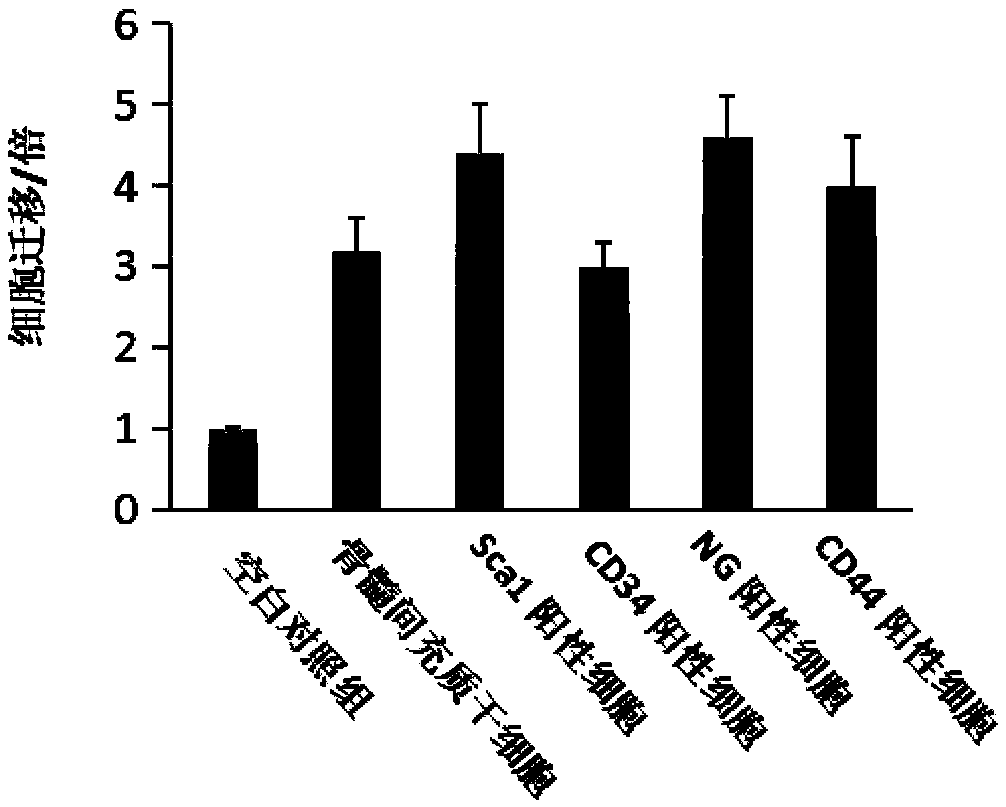
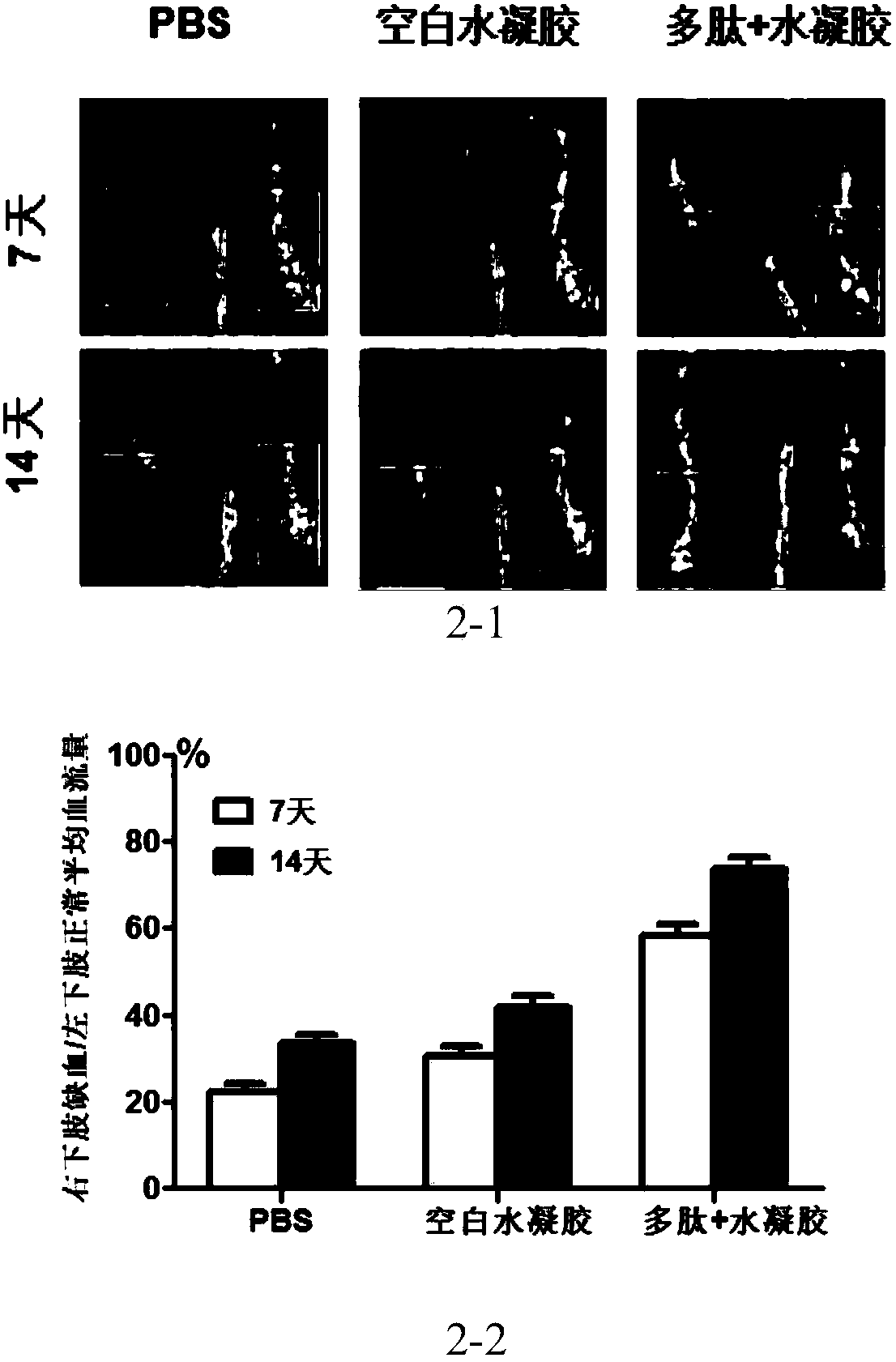
Bioactive polypeptide and application thereof
A technology of biologically active polypeptides and biologically active components, applied to biologically active polypeptides and their application fields, can solve the problems of high cost and low cell survival rate, and achieve the effects of improving blood supply, inducing migration, proliferation and differentiation, and promoting repair.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] In the present invention, there is no special limitation on the method for obtaining the biologically active polypeptide, and the polypeptide synthesis method well known to those skilled in the art can be used for artificial synthesis. Specifically, the solid-phase synthesis method of the biologically active polypeptide of the present invention comprises the following steps:
[0025] (1) The C-terminal of the first amino acid is grafted on the carboxyl resin, and the loading rate is 0.6mmol / g; 20% of piperidine is dissolved in N,N'-dimethylformamide (DMF), and the amino group is removed The Fmoc protecting group; In the present invention, the carboxyl resin includes Wang resin or 2-chlorotriphenyl chloride resin;
[0026] (2) Under the combined action of the condensing agent O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU) and the catalyst N,N-diisopropylethylamine (DIPEA), the carboxyl group of the next amino acid Combine with free amino groups; repeat th...
Embodiment 1
[0098] Solid Phase Synthesis of {L-Met}{L-His}{L-Ser}{L-Pro}G{L-Ala}{L-Asn} Peptides:
[0099] Peptides are synthesized by a well-established solid-phase synthesis (SPPS) procedure using 2-chlorotrityl chloride resin and amino acids with side chains protected by tert-buty groups and amino groups protected by Fmoc. The steps are:
[0100] (1) The C-terminus of the first amino acid was grafted on 2-chlorotrityl chloride resin, and the loading rate was 0.6mmol / g. 20% piperidine is dissolved in N, N'-dimethylformamide (DMF), and the Fmoc protecting group of the amino group is removed;
[0101] (2) Under the joint action of condensing agent O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU) and catalyst N, N-diisopropylethylamine (DIPEA), the carboxyl group of the next amino acid Combine with free amino groups; repeat the above steps, the polypeptide chain will continue to grow;
[0102] (3) Use a certain volume of DMF to repeatedly rinse the resin with the polypeptid...
Embodiment 2
[0106] Solid Phase Synthesis of {D-Met}{L-His}{L-Ser}{L-Pro}G{D-Ala}{D-Asn} Peptides:
[0107] Peptides are synthesized by a well-established solid-phase synthesis (SPPS) procedure using 2-chlorotrityl chloride resin and amino acids with side chains protected by tert-buty groups and amino groups protected by Fmoc. The steps are:
[0108] (1) The C-terminus of the first amino acid was grafted on 2-chlorotrityl chloride resin, and the loading rate was 0.6mmol / g. 20% piperidine was dissolved in N, N'-dimethylformamide (DMF) for removing the Fmoc protecting group of the amino group;
[0109] (2) Under the joint action of condensing agent O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU) and catalyst N, N-diisopropylethylamine (DIPEA), the carboxyl group of the next amino acid Combine with free amino groups; repeat the above steps, the polypeptide chain will continue to grow;
[0110] (3) Use a certain volume of DMF to repeatedly rinse the resin with the polypeptide (5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com