Application of curcumin derivatives in the field of visible light-curing
A technology of curcumin derivatives and curcuminoids, which is applied in the field of organic photofunctional molecule synthesis and visible light photopolymerization, can solve the problems of insensitization of iodonium salt photopolymerization, etc., and achieve good photobleaching properties and ultraviolet-visible absorption wavelength range and light-absorbing ability to improve, the effect of easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
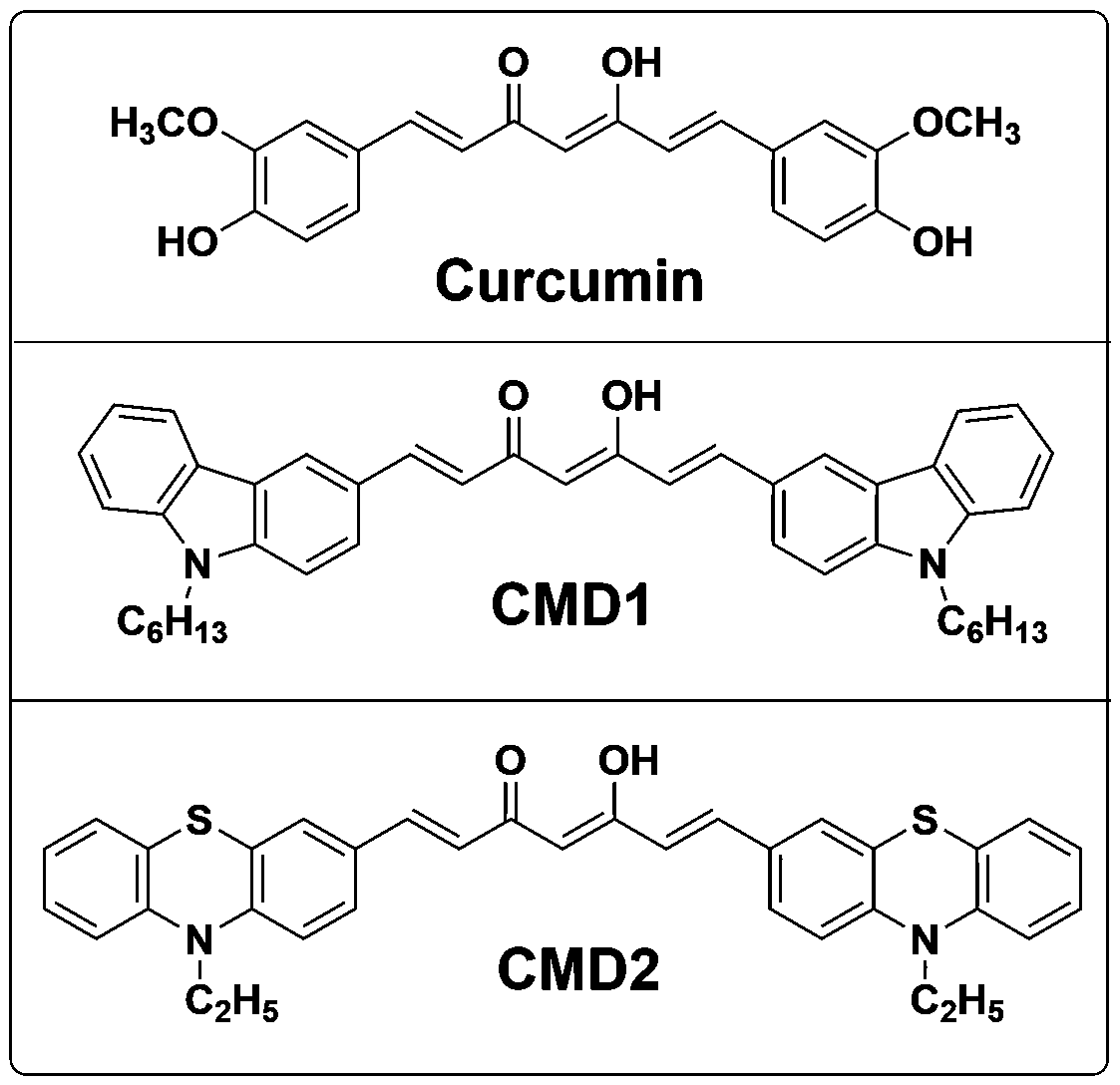
[0043] Synthesis of curcumin derivative CMD1:
[0044]
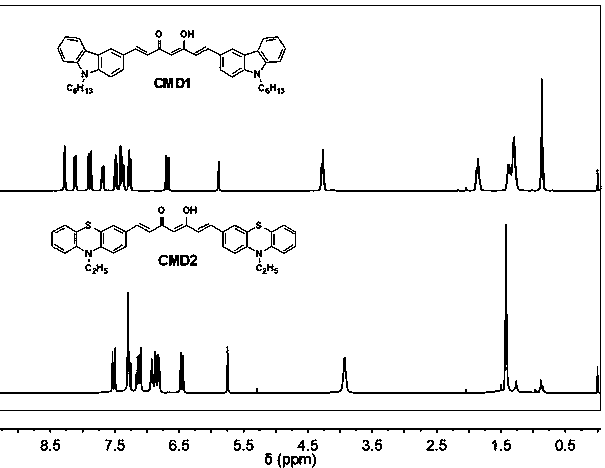
[0045] Add 0.35g (5mmol) diboron trioxide and 0.5g (5mmol) acetylacetone to 10mL of DMF and stir magnetically in an oil bath at 80°C for 30 minutes. Then 9.6 g (20 mmol) of tributyl borate were added and stirring was continued for 30 minutes. Dissolve N-hexylcarbazole aldehyde in 10 mL of DMF and add it into the reaction system, followed by slowly adding 5 mL of a DMF solution containing 2 mmol of n-butylamine dropwise. After the completion of the reaction was monitored by thin-layer chromatography, 1N hydrochloric acid solution was added to adjust the pH value of the system to 5, and the stirring was continued for 1 hour. The resulting product was extracted with ethyl acetate. The obtained organic phase was washed with water, dried and rotary evaporated to remove ethyl acetate, and purified by column chromatography to obtain the product. Red crystals, yield 65%. The hydrogen spectrum is as follows: 1 H NMR (400 ...
Embodiment 2
[0047] Synthesis of curcumin derivative CMD2:
[0048]
[0049] Add 0.35g (5mmol) diboron trioxide and 0.5g (5mmol) acetylacetone to 10mL of DMF and stir magnetically in an oil bath at 80°C for 30 minutes. Then 9.6 g (20 mmol) of tributyl borate were added and stirring was continued for 30 minutes. Dissolve N-ethylphenothiazinaldehyde in 10 mL of DMF and add it into the reaction system, followed by slowly adding 5 mL of a DMF solution containing 2 mmol of n-butylamine dropwise. After the completion of the reaction was monitored by thin-layer chromatography, 1N hydrochloric acid solution was added to adjust the pH value of the system to 5, and the stirring was continued for 1 hour. The resulting product was extracted with ethyl acetate. The obtained organic phase was washed with water, dried and rotary evaporated to remove ethyl acetate, and purified by column chromatography to obtain the product. Dark red crystals, yield 62%. The hydrogen spectrum is as follows: 1 H NM...
Embodiment 3
[0051] The proportion of each component of a visible light initiating system that initiates free radical curing:
[0052] Curcumin derivative CMD1: 0.2 wt%;
[0053] Photoinitiator: 2 wt%;
[0054] Additives: 0.2 wt%.
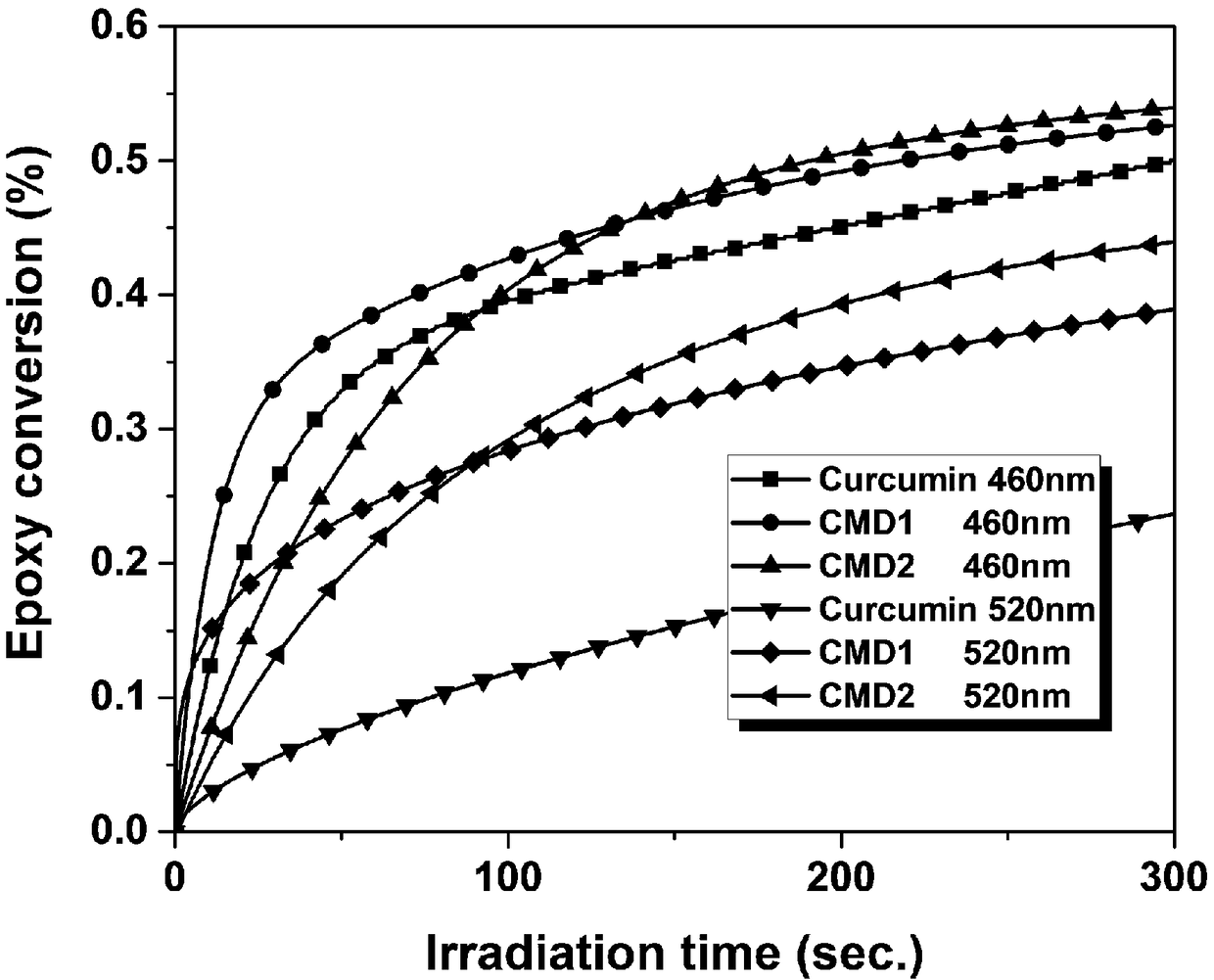
[0055] Prepare the visible light-initiating system according to the above-mentioned ratio. Based on the weight of the radical-type polymerizable monomer as 100%, add the visible-light-initiating system to the free-radical photopolymerizable monomer: TPGDA, and mix thoroughly to obtain a transparent and clear photocuring reaction solution. Add the prepared light-curing system into a rubber ring mold with a thickness of 0.6 mm and a diameter of 1.5 mm, fix it with two clean glass pieces, and irradiate it with blue light (460nm) and green light (520nm) LEDs respectively. Ensure that the distance between the sample and the radiation source is 5cm. In order to ensure the credibility of the experimental results, three NIR tests were performed on each photocuring s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com