The Extraction and Separation Method and Application of Pimaric Acid
A technology of pimaric acid and pinadienoic acid is applied in the separation/purification of carboxylic acid compounds, active ingredients of anhydride/acid/halide, organic chemistry, etc., to achieve the effects of process stability, strong inhibition and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This example is one of the preferred implementations of the extraction and separation of pimaric adienoic acid, and the specific operation process is as follows.
[0037] Take by weighing the dried root bark of 10kg of Acanthopanax chinensis, break into a fine powder through a pulverizer, and the particle size of the crushed material is 3-5mm, and set aside. At room temperature, soak twice with 80L acetone-water (70:30, v:v), each time for 72 hours. After combining the two soaking solutions, use a rotary evaporator to concentrate under reduced pressure to a small volume, and control the concentration temperature not to be higher than 55°C. The obtained concentrate was extracted three times with the same amount of ethyl acetate, and the three extracts were combined. The obtained extract was concentrated to dryness to obtain 700 g of an ethyl acetate extract. Dissolve the extracted portion of ethyl acetate with a small amount of dichloromethane-methanol (2:1, v:v) and a...
Embodiment 2
[0040] The embodiment is one of the preferred implementations of the extraction and separation of the pimaric adienoic acid, and the specific operation process is as follows.
[0041]Take by weighing the dried root bark of 2kg of Acanthopanax chinensis, break into fine powder through a pulverizer, and the particle size of the crushed material is 3-5mm, and set aside. At room temperature, soak twice with 20L acetone-water (80:20, v:v), each time for 72 hours. After combining the two soaking solutions, use a rotary evaporator to concentrate under reduced pressure to a small volume, and control the concentration temperature not to be higher than 55°C. The obtained concentrate was extracted three times with the same amount of ethyl acetate, and the three extracts were combined. The resulting extract was concentrated to dryness to obtain 180 g of an ethyl acetate extract. Dissolve the extracted portion of ethyl acetate with a small amount of dichloromethane-methanol (2:1, v:v) an...
Embodiment 3
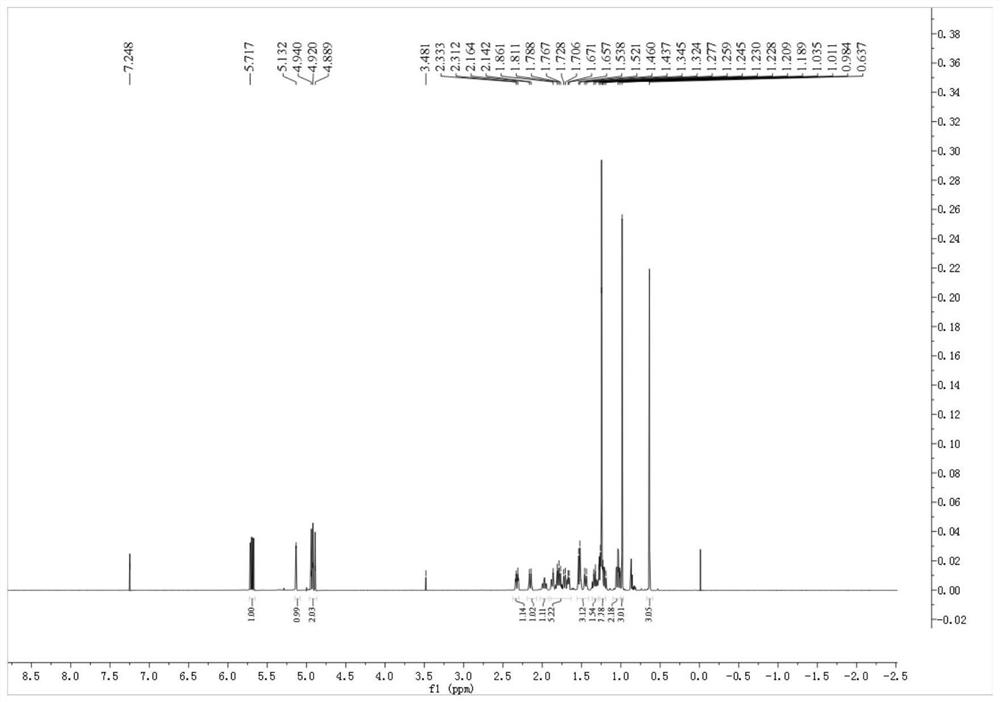
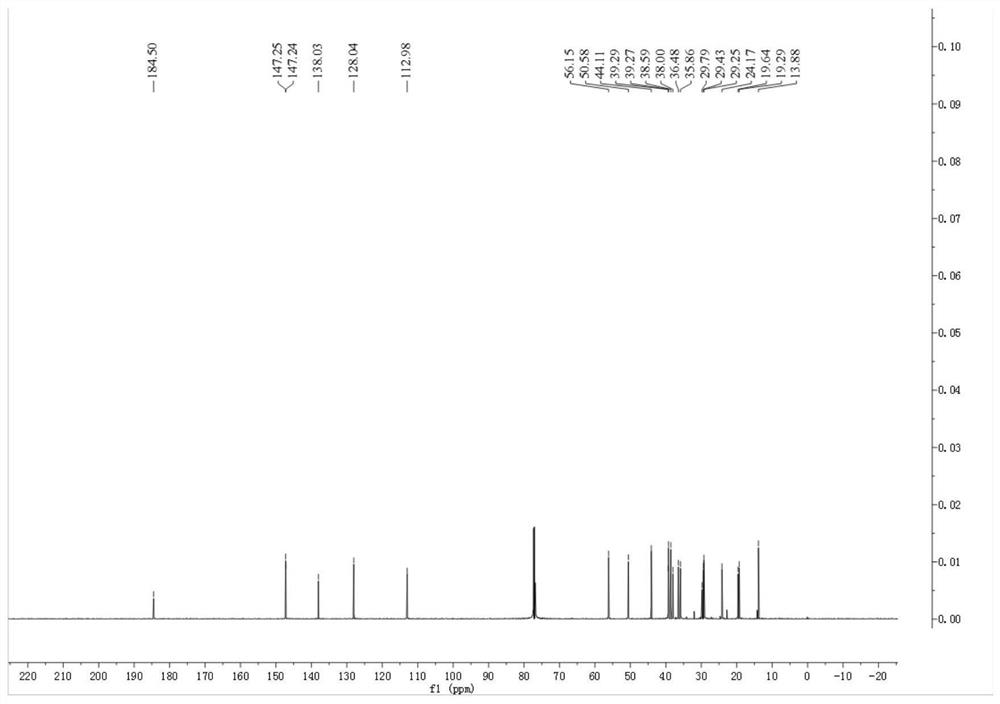
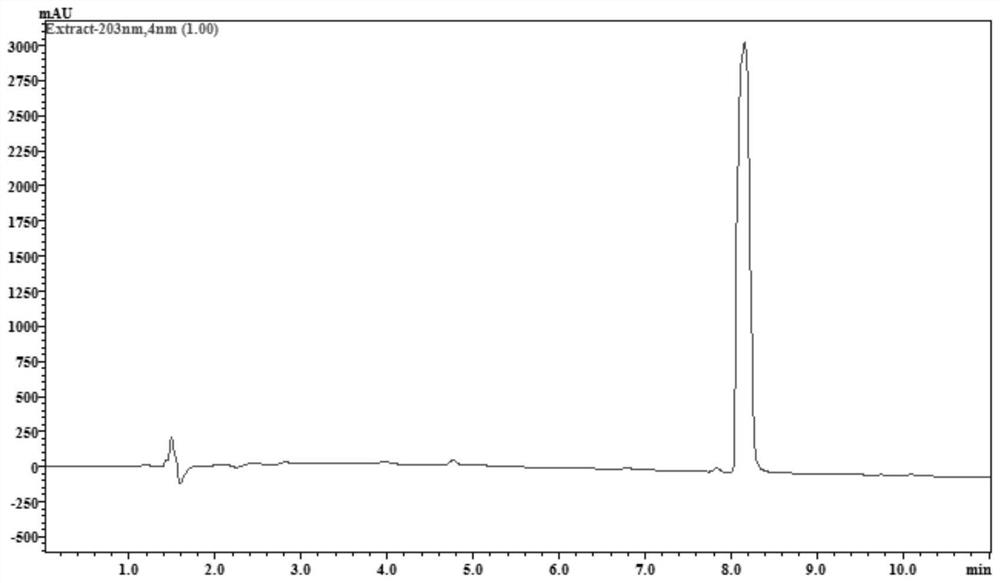
[0044] figure 1 gives the pimaric adienoic acid 1 H-NMR spectrum. figure 2 gives the pimaric adienoic acid 13 C-NMR spectrum. image 3 The HPLC spectrum of the pimaric acid is given. It has been confirmed that the pimaric dienoic acid extracted and separated in Examples 1 and 2 has the structure of formula (I), and its relevant physical and chemical parameters and structural data are as follows.
[0045] Molecular formula: C 20 h 30 o 2 ;
[0046] Molecular weight: 302.45;
[0047] Properties: white massive crystal;
[0048] Solubility: easily soluble in dichloromethane, chloroform, acetone, almost insoluble in water;
[0049] Melting point: 162–163°C;
[0050] Optical rotation [α]25D=–129°(c=0.8, CHCl 3 );
[0051] 1 H-NMR (600MHz, CDCl 3 )δ H :5.72(1H,dd,J=17.1,10.4Hz,H-15),5.13(1H,br s,H-14),4.93(1H,dd,J=10.4,2.0Hz,H-16a),4.90 (1H, dd, J = 17.1, 2.0 Hz, H-16b), 1.24 (3H, s, H-17), 0.98 (3H, s, H-18), 0.64 (3H, H-20).
[0052] 13 C-NMR (CDCl 3 ,150MHz)δ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com