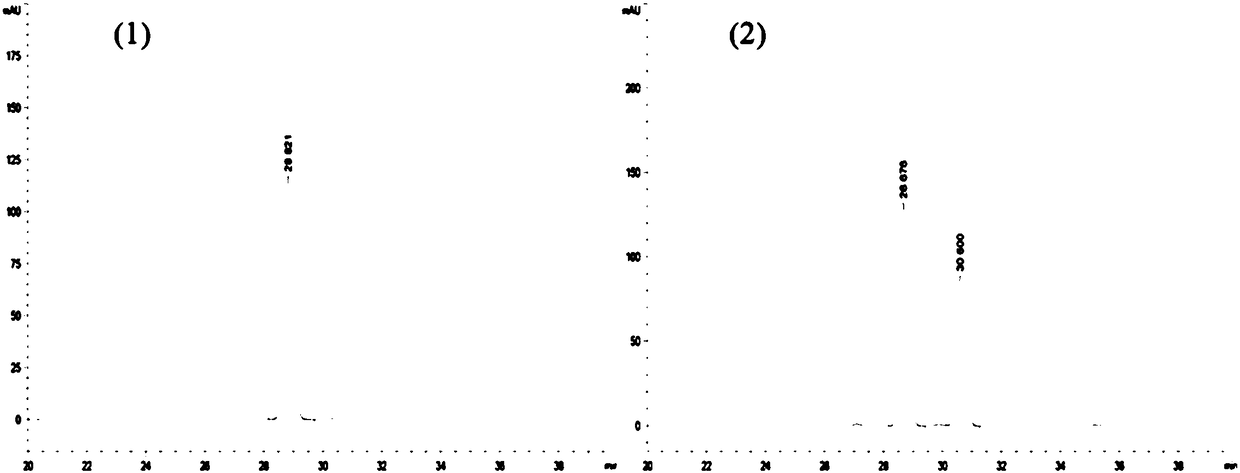
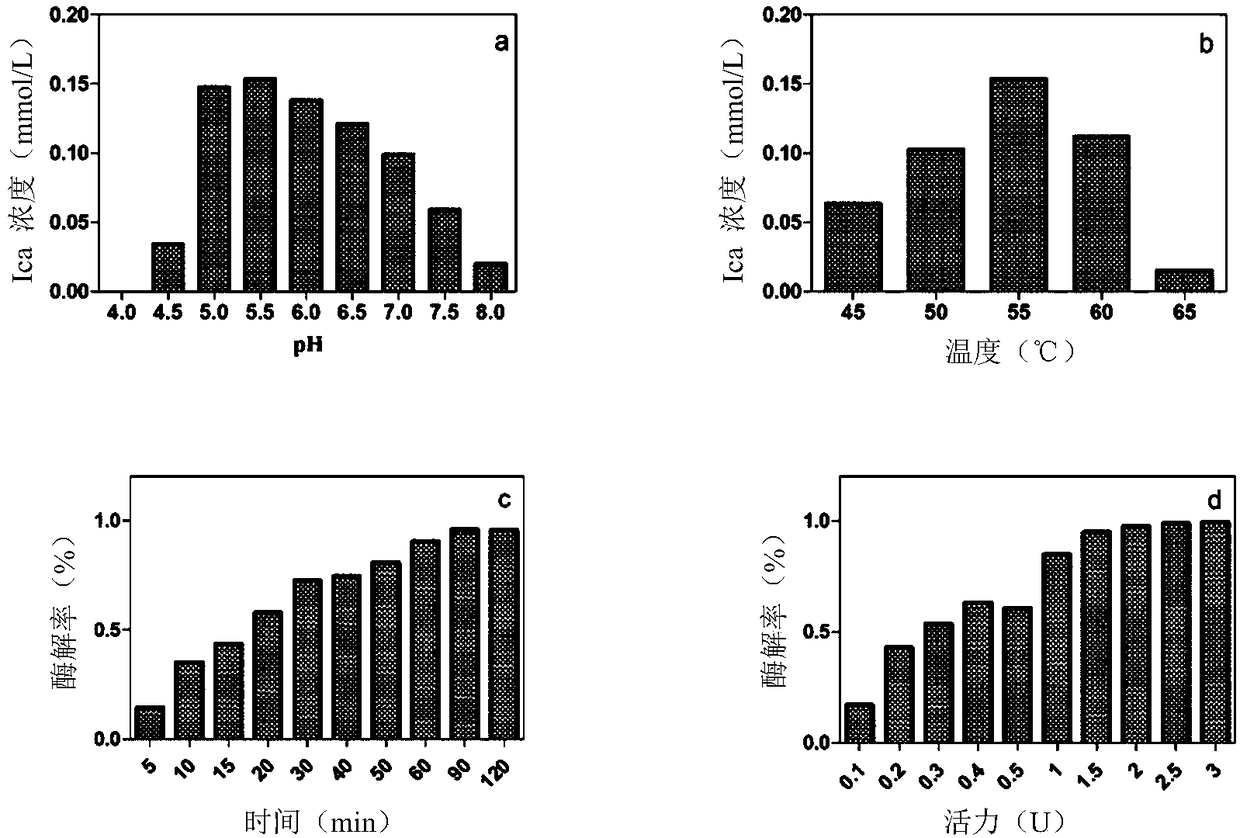
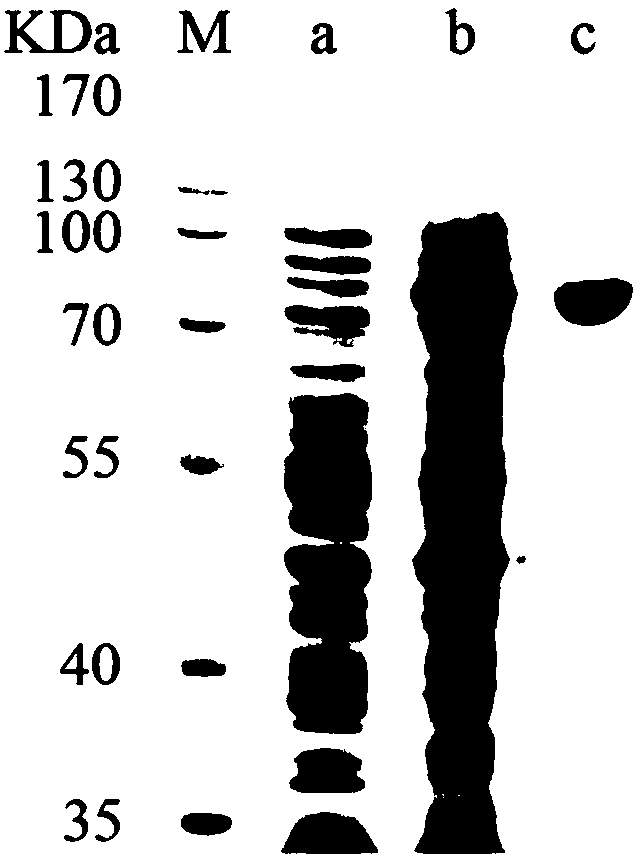Alpha--L-rhamnosidase and application thereof
A technology of rhamnosidase and icariin, which is applied in the fields of genetic engineering and enzyme engineering, and can solve problems such as ineffective degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Cloning of α-L-rhamnosidase BtRha gene and construction of expression vector
[0030] Using the Bacteroidesthetaiotaomicron VPI-5482 genome purchased from DSMZ (https: / / www.dsmz.de / ) as a template, degenerate primers P1 and P2 were designed according to the amino acid sequence of bacterial rhamnosidase on NCBI , for the upstream and downstream primers to amplify the BtRha gene fragment, use Ex Taq polymerase (purchased from Takara Company) to prepare 50 μL reaction solution at the recommended ratio for fragment amplification, the PCR reaction conditions are 95 ° C, 5 min; 35 cycles (95 ° C, 30s; 52°C, 30s; 72°C, 2min 20s); 72°C, 10min; the reaction was stopped and kept at 4°C. PCR amplification products were purified by gel recovery kit. To obtain the Bacteroides thetaiotaomicron VPI-5482 α-L-rhamnosidase BtRha gene, the resulting PCR amplified product and pET28a were digested with Nco I and Xhol I respectively, and the fragments recovered were combined with pET-28a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com