Application of Cap-TFlg protein in preparing PCV2 vaccine
A protein and vaccine technology, applied in biochemical equipment and methods, using vectors to introduce foreign genetic material, viral peptides, etc., can solve the problem that the immune effect needs to be further improved, achieve good immunogenicity and protection, and enhance immune response. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
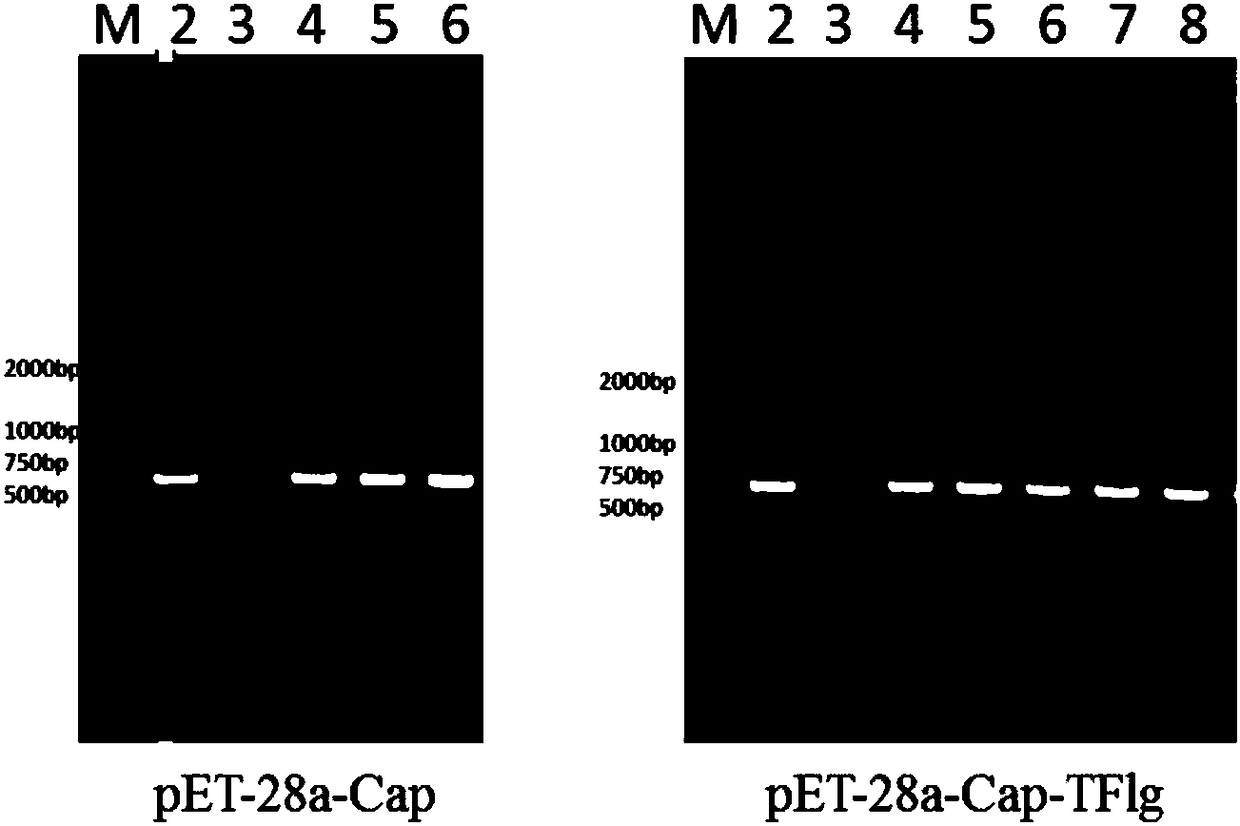
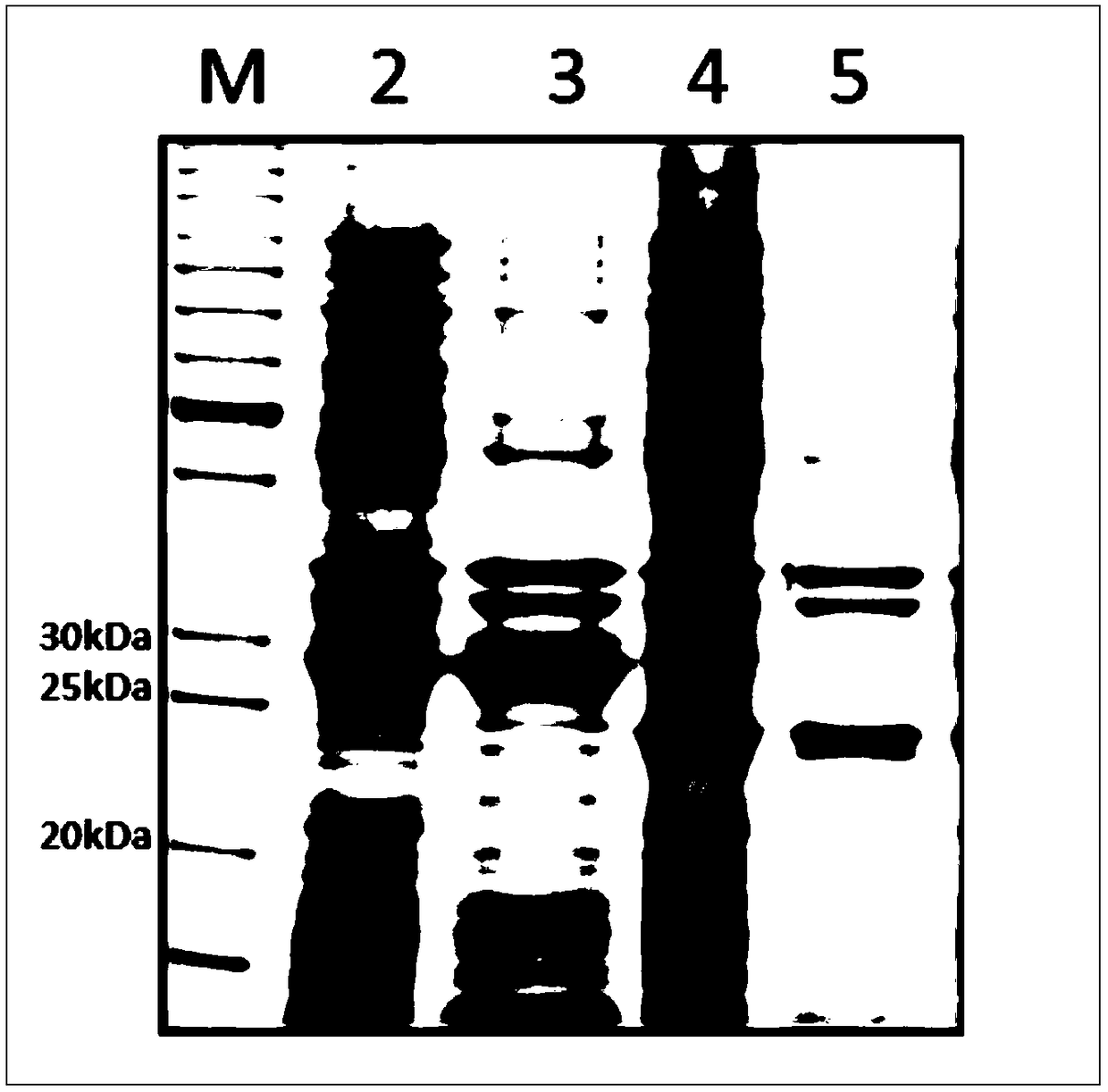
[0029] Embodiment 1: A kind of vector construction of expressing Cap and Cap-TF1g protein
[0030] Step 1: Cap and Cap-TFlg genes are artificially synthesized, and their nucleotide sequences are shown in SEQ ID NO.1 and SEQ ID NO.2, with an Nco I restriction site at the 5' end and a Hind at the 3' end III restriction site.
[0031] Step 2: Use the pET-28α (preserved in our laboratory) vector as the backbone, use Nco I and Hind III to digest and recover the backbone fragment, use Nco I and Hind III to digest and recover the Cap-TFlg fragment, and then use ligase to Ligated overnight at 16°C, and the ligated product was transformed into E. coli From the E. coli competent cells of EZ10 strain, colonies were finally picked and dissolved in 10 µL medium for colony PCR identification. The positive recombinants were extracted into plasmids, identified by enzyme digestion and sequenced, and finally the transfer vector pBac-Cap3 was obtained.
[0032] Among them, PCR identification ...
Embodiment 2
[0049] Example 2: Soluble expression of Cap and Cap-TFlg proteins
[0050] Step 1: Transfer the pre-expressed and identified strains to a large Erlenmeyer flask (capacity 1000ml) with 200ml LB plus antibiotics (such as kana antibiotics, the final concentration is 50ug / ml). The transfer ratio is generally 1:100. Culture conditions: 37°C, 200rpm / min for 3-4 hours.
[0051] Step 2: When the OD600 value reaches 0.4~0.6, add lactose (final concentration 1%) to induce expression. Conditions for inducing expression: culture at 37°C, 160 rpm / min for 6 hours.
[0052] Step 3: Use a 200ml centrifuge cup to collect the bacteria, centrifuge at 6000rpm at 4°C for 10 minutes, and discard the supernatant. Xiangyi centrifuge model: GL-21M
[0053] Step 4: Add 50ml of buffer solution (20mmol / L Tris HCl, 0.5mol / L NaCl, pH7.9) into the centrifuge cup, and repeatedly pipette with a 5ml pipette tip to disperse the cells evenly in the buffer solution.
[0054] Step 5: The resuspended bacteria ...
Embodiment 3
[0058] Embodiment 3: Cap and Cap-TFlg protein VLPs electron microscope observation sample preparation
[0059] After 10~30s of plasma cleaning on the copper mesh, the carbon side faces up; spread a piece of sealing film on the ice box to cool; put the copper mesh on the ice sealing film; drop samples, ultrapure water, and PTA staining on the sealing film respectively. 20 μl each solution; stand for 1-2 minutes, cool the copper grid, sample, ultrapure water, and PTA; buckle the carbon surface of the copper grid on the sample droplet to absorb the sample for 1 minute, and use filter paper to contact the copper grid vertically to absorb excess Liquid, until no residual liquid can be seen, continue to absorb water for 10s; buckle the copper mesh carbon surface on the PTA droplet to dye for 0.5-~1 minute, and dry it with filter paper; place it on the filter paper, dry it naturally in a cool place, and then use it Electron microscope observation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com