Preparation method and application of rare earth cerium-quantum dot coordination polymer double-emission fluorescent probe
A dual-emission fluorescence and coordination polymer technology, applied in the field of fluorescence sensing, achieves the effects of good selectivity, improved accuracy, and reduced detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of rare earth cerium-quantum dot coordination polymer dual emission fluorescent probe
[0033] (1) Add 30 μL of 10 mM adenosine triphosphate (ATP) to 300 μL of 50 mM pH 7.4 Tris-HCl buffer solution, mix well, and then add 500 μL of 40 nM carboxy quantum dots (QDs ) and 200μL 4mM Ce(NO 3 ) 3 Solution, stirred slowly at room temperature for 2 minutes, forming a white turbid solution;
[0034] (2) Centrifuge the white turbid liquid at 16500rpm for 10min to obtain a white precipitate, wash it three times with ultrapure water, resuspend the white precipitate in 1mL of ultrapure water, and make a rare earth cerium-quantum dot coordination polymer double emission Fluorescent probes (hereinafter referred to as Ce-QDs CPNs).
Embodiment 2
[0036] Microscanning of Ce-QDs CPNs
[0037] The morphology of the Ce-QDs CPNs prepared in Example 1 was characterized by scanning electron microscope (SEM), the results are as follows figure 1 shown.
[0038] From figure 1 It can be seen that the Ce-QDs CPNs prepared by the method of the present invention are composed of spherical particles with a diameter of about 10 nm to 30 nm. This is mainly due to the fact that the ATP molecule contains a phosphate group with multi-coordinating ability, which converts the rare earth Ce 3+ Rare earth coordination polymers were formed through coordination with carboxylated quantum dots (QDs) in Tris-HCl buffer solution.
Embodiment 3
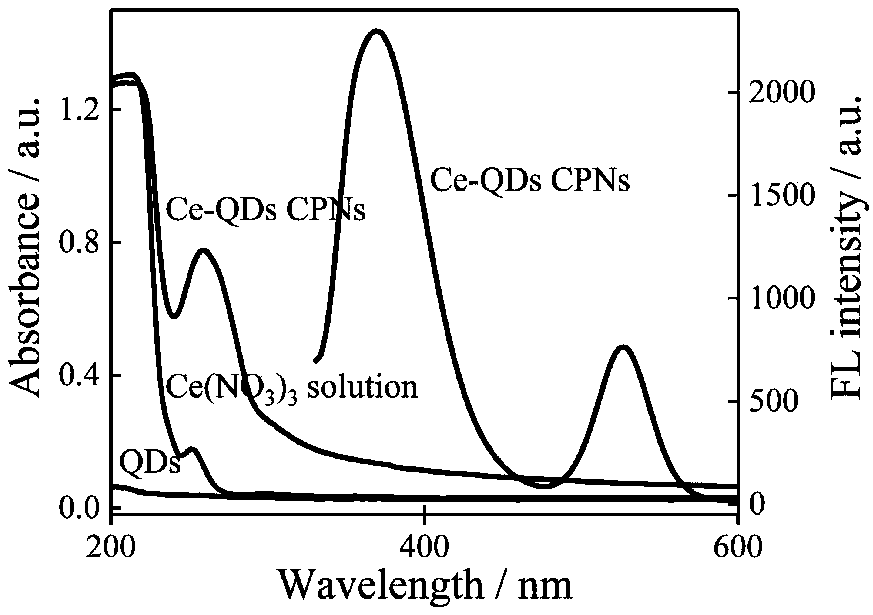
[0040] QDs, Ce(NO 3 ) 3 , UV spectra of Ce-QDs CPNs and fluorescence spectra of Ce-QDs CPNs.
[0041] 2nM QDs solution, 0.08mM Ce(NO 3 ) 3 ·6H 2 UV-Vis absorption spectra of O solution and 0.08 mM Ce-QDs CPNs solution. The fluorescence emission spectrum of the Ce-QDs CPNs solution at the excitation wavelength of 310 nm was measured by a fluorescence spectrometer. The result is as figure 2 shown.
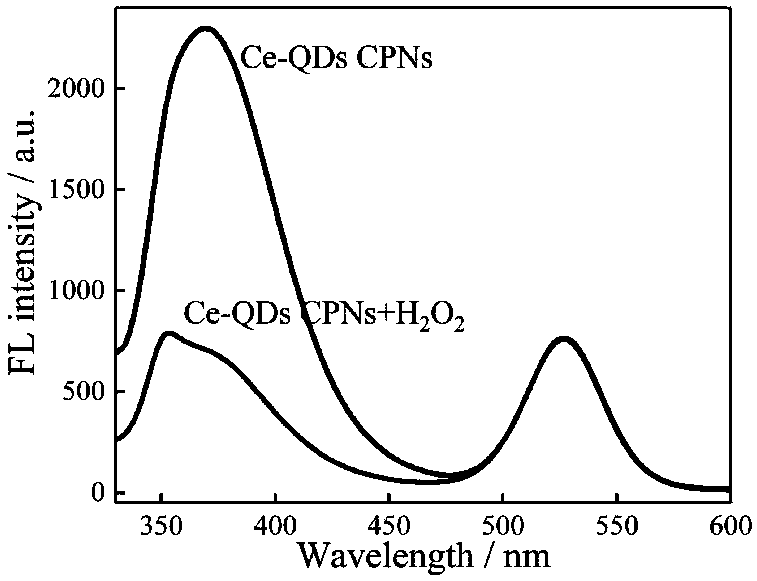
[0042] From figure 2 It can be seen from the UV-visible absorption spectrum that Ce(NO 3 ) 3 ·6H 2 The characteristic ultraviolet absorption peak of O solution is 252nm, QDs has almost no ultraviolet absorption peak, and Ce-QDs CPNs has a strong and broad absorption peak at 260nm. Under the excitation wavelength of 310nm, the Ce(III) and QDs fluorescence peaks of Ce-QDs CPNs appeared at 369nm and 525nm, respectively. Ce by spectrometry 3+ , ATP and QDs self-assembly behavior in Tris-HCl buffer solution was characterized. Ce-QDs CPNs at 1710(νP-OH), 1480(νN7-C8), 1258(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com