Construction method for simultaneously expressing two foreign protein vectors TRVe2 and application
A technology of exogenous protein and construction method, which is applied in the direction of using vectors to introduce foreign genetic material, recombinant DNA technology, etc., and can solve problems such as interference with biological functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
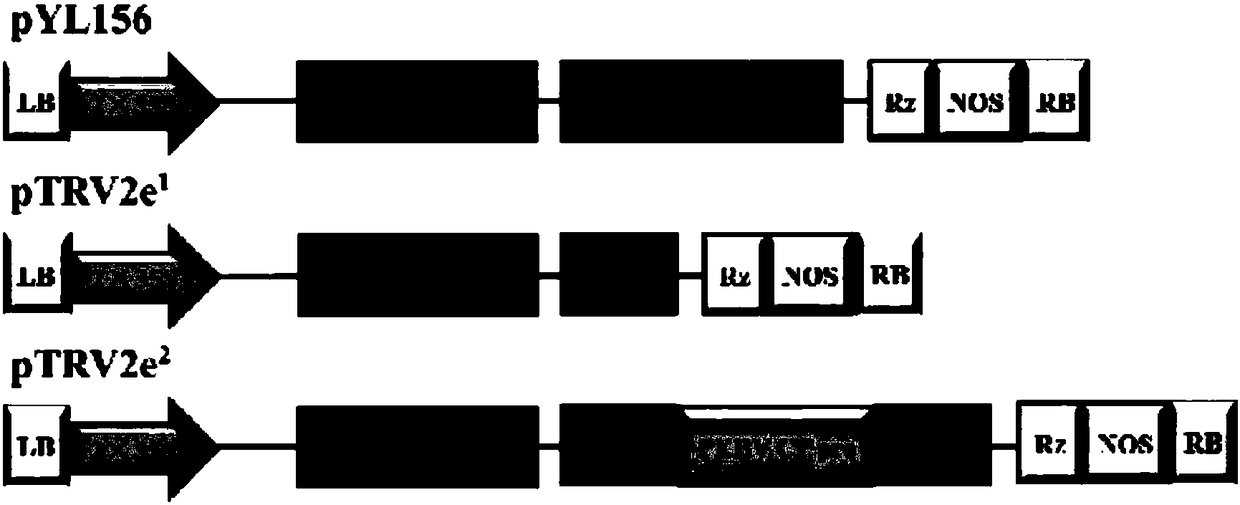
[0049] Embodiment 1, the construction of TRV expression vector
[0050] Using the plasmid pYL156 as a template, P1 / P2 was amplified by PCR with primers. After the target product was recovered by tapping the rubber, it was double-digested with HindⅢ / EcoRI. At the same time, pYL156 was double-digested with HindⅢ / EcoRI. Ligation and transformation of PCR product fragments by enzyme digestion to obtain recombinant plasmid pTRV2e 1 . The sequences of the primers P1 and P2 were cccAAGCTTGCATGCCTGCAG (AAGCTT was HindⅢ restriction site) and cGAATTCtctagaCTCGAGacgcgtAAGCCACTTCCTAAGTAATTCGTGCcTTGCGAAACTCAAATGC (GAATTC was EcoRI, tctaga was XbaI, CTCGAG was XhoI and acgcgt was MluI).
[0051] The PCR amplification system is: 5×Q5 reaction buffer 8μL, dNTP (2.5mM) 3.2μL, P1 / P2 (10μM) 2μL each, template pYL156 10ng, Q5polymerase (1U / μL) 0.4μL, ddH 2 O was added to 40 μL; the PCR reaction program was: 98°C for 3 min, 98°C for 10 s, 56°C for 15 s, 72°C for 60 s, 35 cycles, 72°C for 5 min. ...
Embodiment 2
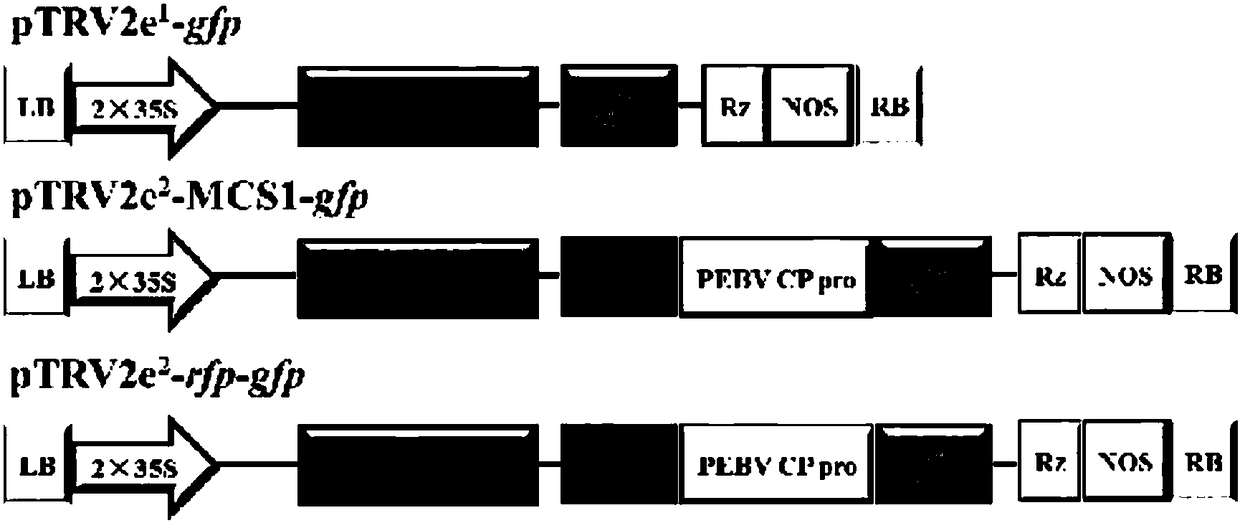
[0055] Embodiment 2, pTRV2e vector construction expressing GFP and RFP
[0056] gfp基因大小为720bp,序列为:atgagtaaaggagaagaacttttcactggagttgtcccaattcttgttgaattagatggtgatgttaatgggcacaaattttctgtcagtggagagggtgaaggtgatgcaacatacggaaaacttacccttaaatttatttgcactactggaaaactacctgttccatggccaacacttgtcactactttctcttatggtgttcaatgcttttcaagatacccagatcatatgaagcggcacgacttcttcaagagcgccatgcctgagggatacgtgcaggagaggaccatcttcttcaaggacgacgggaactacaagacacgtgctgaagtcaagtttgagggagacaccctcgtcaacaggatcgagcttaagggaatcgatttcaaggaggacggaaacatcctcggccacaagttggaatacaactacaactcccacaacgtatacatcatggccgacaagcaaaagaacggcatcaaagccaacttcaagacccgccacaacatcgaagacggcggcgtgcaactcgctgatcattatcaacaaaatactccaattggcgatggccctgtccttttaccagacaaccattacctgtccacacaatctgccctttcgaaagatcccaacgaaaagagagaccacatggtccttcttgagtttgtaacagctgctgggattacacatggcatggatgaactatacaaatag。
[0057] Use primers to perform PCR amplification on P5 / P6, and the target product of gfp gene is recovered by tapping rubber, cleaned and recovered after EcoRI / MluⅠ double enz...
Embodiment 3
[0062] Embodiment 3, Agrobacterium transformation, activation and expanded culture of TRV2-related vectors
[0063] Take 100uL of Agrobacterium GV3101 competent cells and put them into 1.5mL sterilized centrifuge tubes pre-cooled on ice, and add 100ng of plasmid pTRV2e respectively 1 , pTRV2e 2 , pTRV2e 2 -MCS1-GFP and pTRV2e 2 -rfp-gfp, and gently blow and mix, place on ice for 30min; 42°C water bath for 1min, place in liquid nitrogen for 1min, then place on ice for 2min; add 500uL LB liquid medium without antibiotics, shake at 28°C, 220rpm Medium-shake culture for 3-4 hours; respectively take 100uL of the above four bacterial culture solutions and spread evenly on LB solid plates containing (30mg / L Rifampicin, 50mg / L Gentamycin and 50mg / L Kanamycin), and culture in a 28°C incubator 12~16h. Pick the above pTRV2e respectively 1 , pTRV2e 2 , pTRV2e 2 -MCS1-GFP and pTRV2e 2 -rfp-gfp single colony and 20uL Agrobacterium pTRV1 and pYL156 glycerol bacteria stored in minus 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com