Iodixanol and synthesis method thereof
A technology of iodixanol and its synthetic method, which is applied in the field of polymers, can solve the problems of low yield and cumbersome post-treatment in reaction route 1, and achieve the effects of low reaction cost, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The synthetic method of iodixanol provided by the embodiments of the present invention comprises:
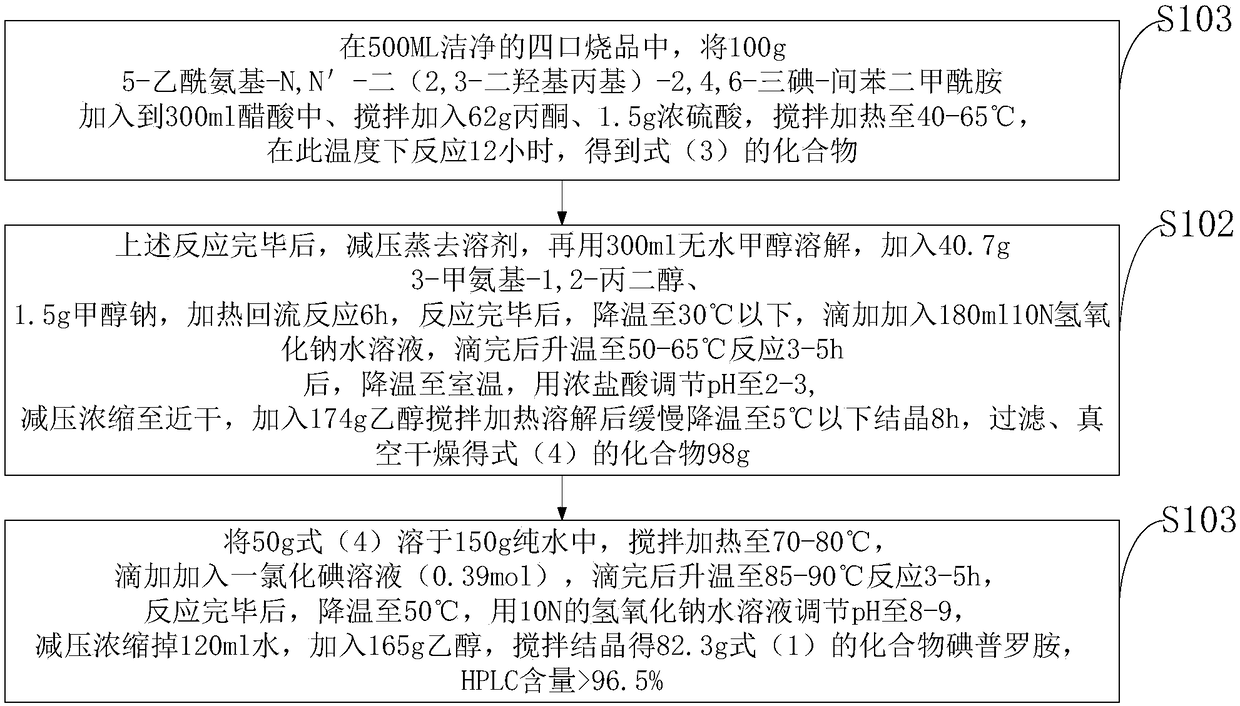
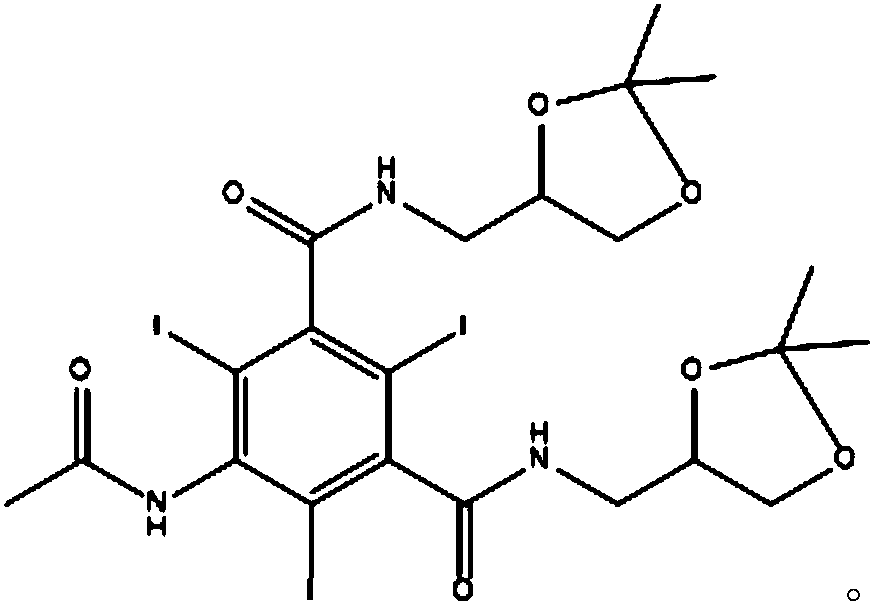
[0044] Step 1: Add 100g of 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide to 500ML clean four-port burnt product Add it into 300ml of acetic acid, stir and add 62g of acetone, 1.5g of concentrated sulfuric acid, stir and heat to 40°C, and react at this temperature for 12 hours to obtain the compound of formula (3);
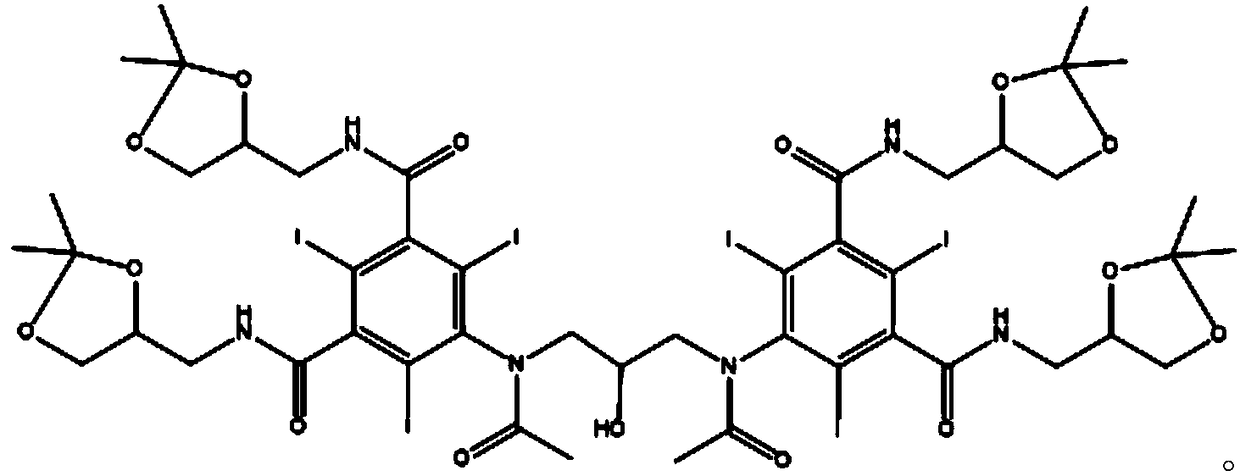
[0045] Step 2: After the above reaction is completed, distill off the solvent under reduced pressure, then dissolve it with 300ml of anhydrous methanol, add 40.7g of 3-methylamino-1,2-propanediol and 1.5g of sodium methoxide, and heat under reflux for 6 hours. After the reaction is completed, cool down To below 30°C, add 180ml of 10N sodium hydroxide aqueous solution dropwise, after dripping, raise the temperature to 50°C for reaction 3, cool down to room temperature, adjust the pH to 2 with concentrated hydrochloric acid, concentrate ...
Embodiment 2
[0048] The synthetic method of iodixanol provided by the embodiments of the present invention comprises:
[0049] Step 1: Add 100g of 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide to 500ML clean four-port burnt product Add it to 300ml of acetic acid, stir and add 62g of acetone, 1.5g of concentrated sulfuric acid, stir and heat to 65°C, and react at this temperature for 12 hours to obtain the compound of formula (3);
[0050]Step 2: After the above reaction is completed, distill the solvent off under reduced pressure, then dissolve it with 300ml of anhydrous methanol, add 40.7g of 3-methylamino-1,2-propanediol, 1.5g of sodium methoxide, and heat to reflux for 6 hours. After the reaction is completed, cool down When the temperature is below 30°C, add 180ml of 10N sodium hydroxide aqueous solution dropwise, after dropping, raise the temperature to 65°C and react for 3-5 hours, then cool down to room temperature, adjust the pH to 3 with concentrated hydro...
Embodiment 3
[0053] The synthetic method of iodixanol provided by the embodiments of the present invention comprises:
[0054] Step 1: Add 100g of 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide to 500ML clean four-port burnt product Add to 300ml of acetic acid, stir and add 62g of acetone, 1.5g of concentrated sulfuric acid, stir and heat to 55°C, and react at this temperature for 12 hours to obtain the compound of formula (3);
[0055] Step 2: After the above reaction is completed, distill the solvent off under reduced pressure, then dissolve it with 300ml of anhydrous methanol, add 40.7g of 3-methylamino-1,2-propanediol, 1.5g of sodium methoxide, and heat to reflux for 6 hours. After the reaction is completed, cool down When the temperature is below 30°C, add 180ml of 10N sodium hydroxide aqueous solution dropwise, after the dropwise temperature rises to 55°C and reacts for 3-5 hours, then cools down to room temperature, adjusts the pH to 2.5 with concentrated hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com