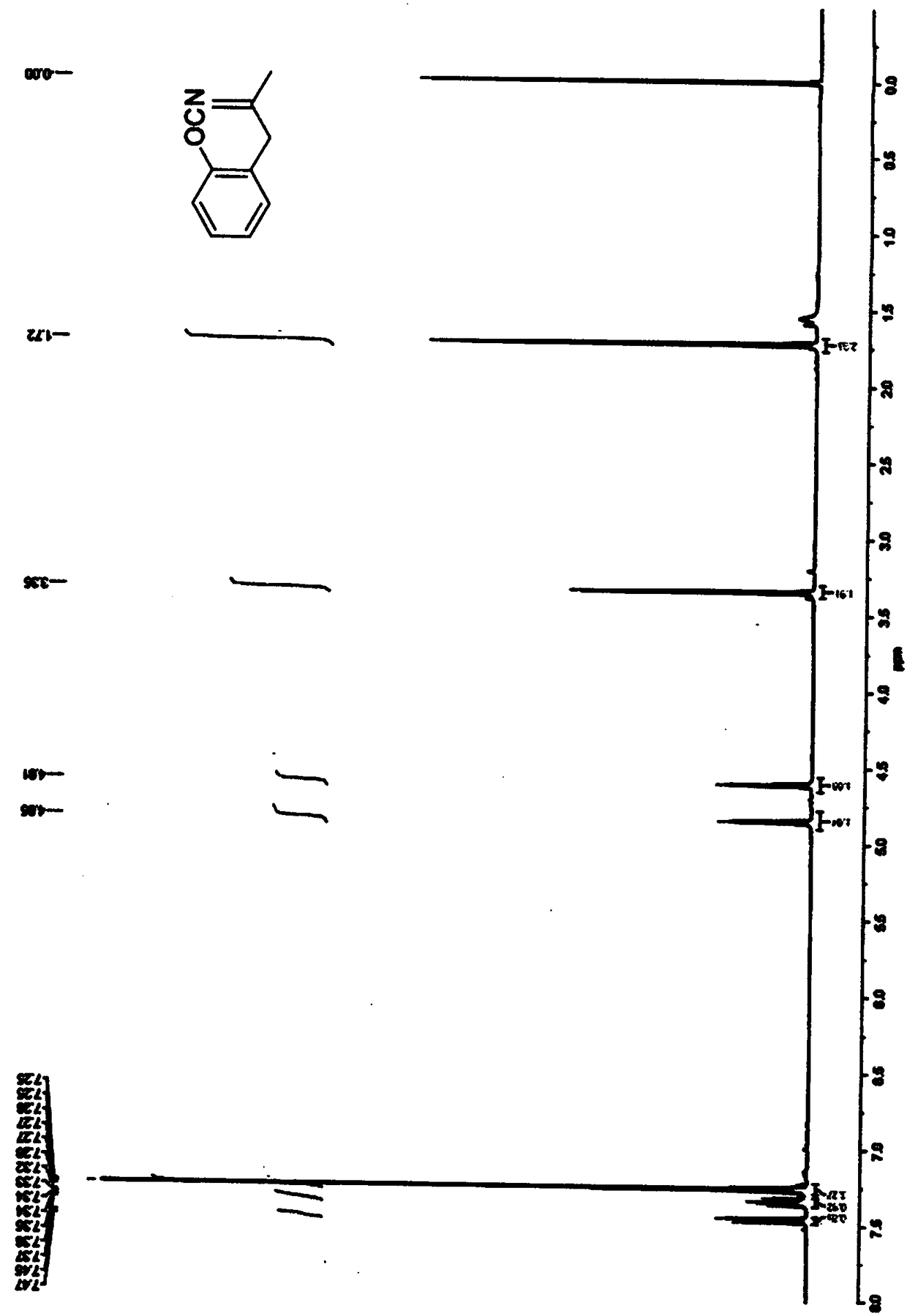
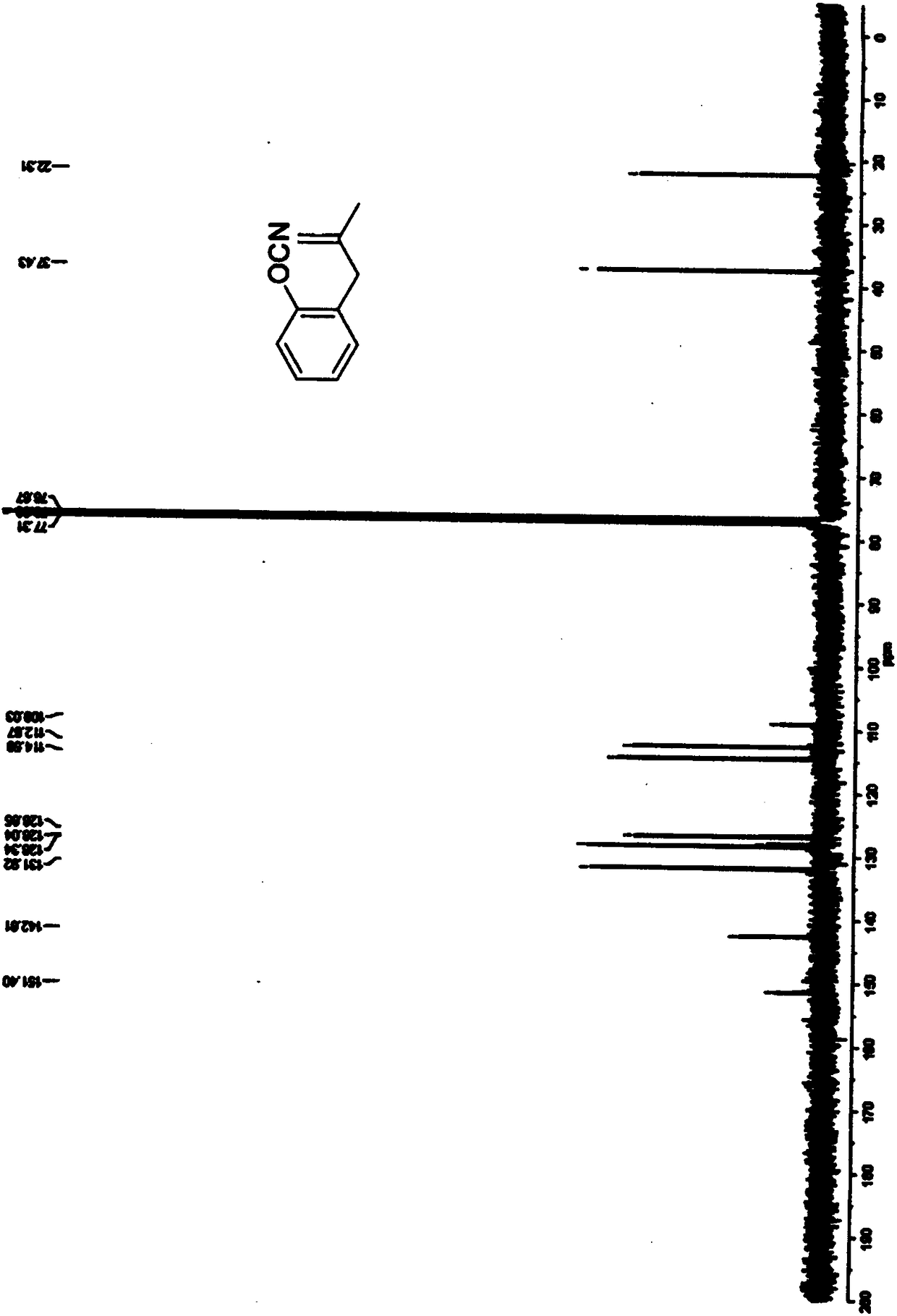
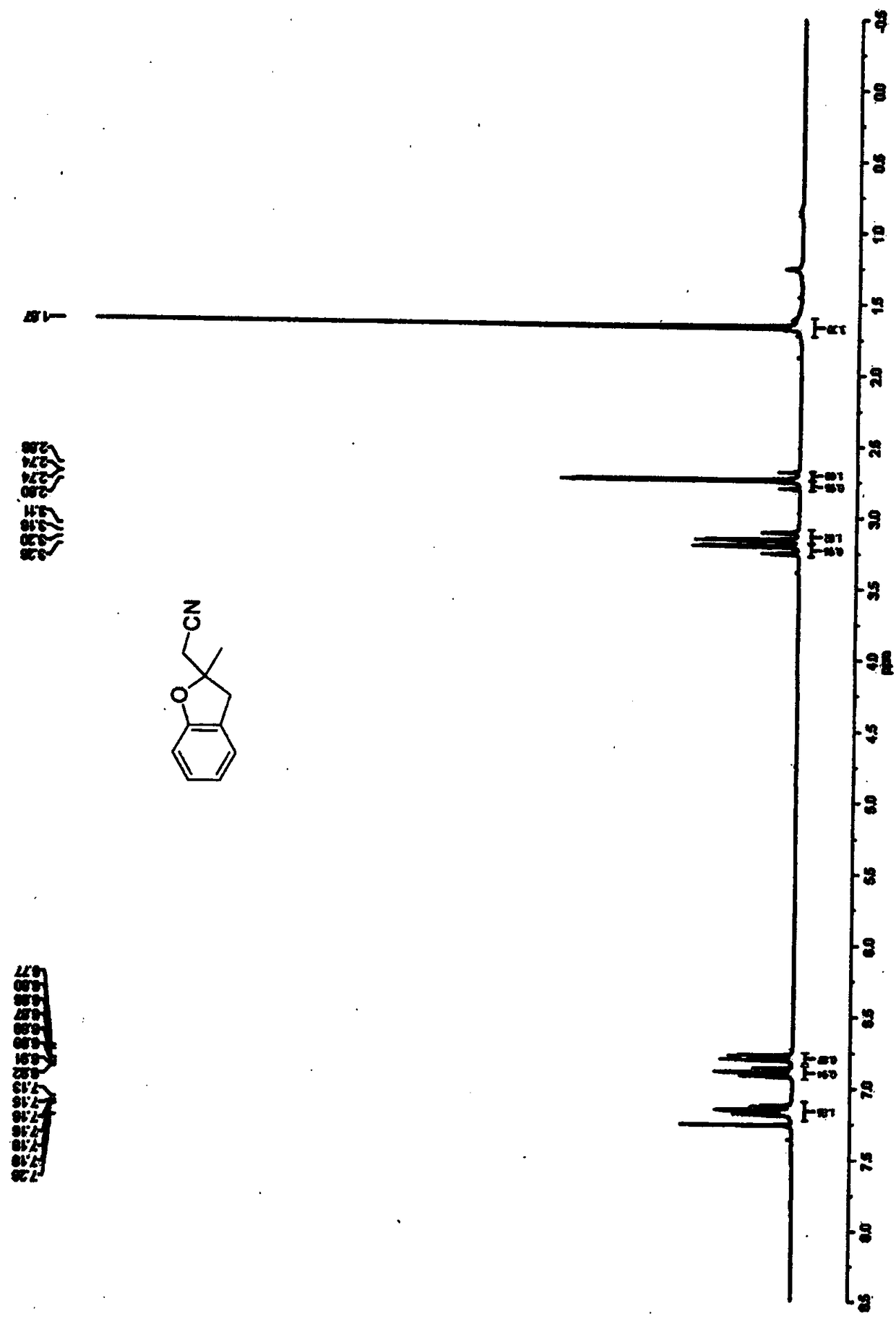
Method for compounding benzofuran derivatives by adding C-O bonds into olefin molecules through non-metallic Lewis acid catalysis
A Lewis acid catalyzed olefin and benzofuran technology, which is applied in organic chemistry and other fields, can solve problems such as transition metal residues, and achieve the effect of avoiding pollution and eliminating post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of benzofuran derivative A in this embodiment includes the following steps:
[0028] (1) Preparation of olefins:
[0029] The reaction formula is:
[0030]
[0031] 12 grams of potassium carbonate, 8.6 mL of methallyl chloride and 7.6 grams of phenol (compound 1) were added to 20.0 mL of acetone solution being stirred, and the resulting mixture was refluxed overnight at room temperature, then 100 mL of water was added, and then Extracted with ether (12 x 10ml). The collected organic layer was washed with 2M NaOH (3x80 mL), washed with MgSO 4 Drying, filtration and concentration in vacuo yielded 4.8 g of intermediate compound 2, which was used without purification.
[0032] 4.8 g of compound 2 was dissolved in 4.0 mL of N,N-dimethylaniline, and the mixture was refluxed at 205° C. for 5 hours in a nitrogen atmosphere. After cooling to room temperature, 400 mL of diethyl ether was added, and the reaction mixture was washed with 1M HCl (3x200 mL). The ...
Embodiment 2
[0040] The synthesis of benzofuran derivative B in this embodiment includes the following steps:
[0041] (1) Preparation of olefins:
[0042] The reaction formula is:
[0043]
[0044] 12 g of potassium carbonate, 8.0 mL of methallyl chloride and 8.0 g of p-methoxyphenol (Compound 1) were added to 16.0 mL of stirred acetone solution, and the resulting mixture was refluxed overnight at room temperature, after which 100 mL of water was added , then extracted with ether (12 x 10ml). The collected organic layer was washed with 2M NaOH (3x80 mL), washed with MgSO 4 Drying, filtration and concentration in vacuo yielded 8.1 g of intermediate compound 2, which was used without purification.
[0045] 8.0 g of intermediate product 2 was dissolved in 35 mL of N,N-dimethylformamide DMF solvent, and the mixture was refluxed at 240 °C for 80 minutes under microwave conditions in a nitrogen atmosphere. Cool to room temperature and concentrate under reduced pressure. The crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com