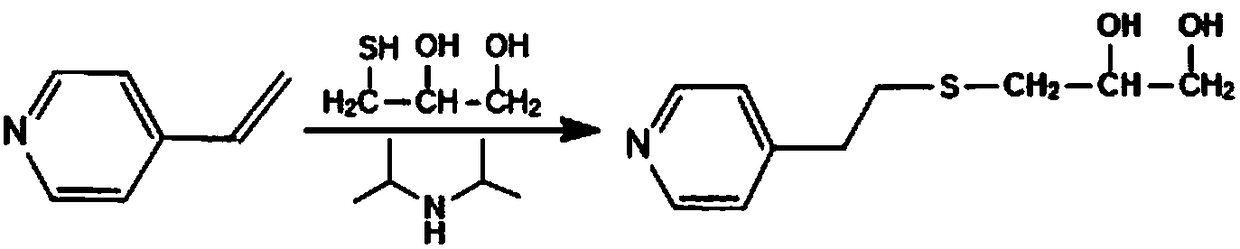
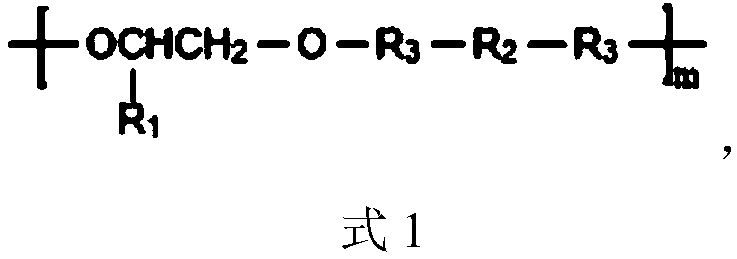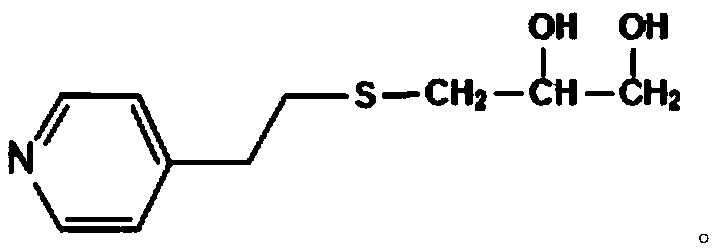PH sensitive medical polyurethane urea material and preparation method thereof
A polyurethane urea and sensitive technology, applied in the field of biological material preparation, can solve the problems of toxicity, use restriction, complex preparation method, etc., and achieve the effects of low cost, avoiding the generation of thrombus, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The preparation method of LBL is: under the protection of dry nitrogen and mechanical stirring, add 1,4-butanediamine dropwise to L-lysine diisocyanate (-NCO:-NH 2 =8:1, molar ratio), after reacting at room temperature for 2h, add four times the volume of n-hexane to the reaction product, after stirring evenly, obtain a white solid by suction filtration, wash with n-hexane repeatedly until the filtrate IR detects that there is no -NCO absorption Peak (2270cm -1 ), vacuum-dried to constant weight to obtain white powder LBL.
[0068] LBL's 1 H NMR structure characterization results:
[0069] 1 H NMR (DMSO-D6, 400MHz, ppm): 1.27-1.32 (m, 10H, CH 3 CH 2 and C H 2 CH 2 CHNCO), 1.52-1.55(m,8H, CH 2 CH 2 NH),1.75(q,4H, CH 2 CHNCO),3.08-3.16(t,8H, CH 2 NH), 4.08-4.15 (m, 6H, CH-NCO and CH 3 C H 2 ), 5.95-6.04 (br, N H ).
Embodiment 1
[0071] Under the protection of dry nitrogen, 5.370g (30mmol) terminal dihydroxypyridine compound (VP-(OH) 2 ) with 5.0g (5mmol) polyethylene glycol (PEG, M n =1000) were mixed, N,N-dimethylformamide (DMF) was added to dissolve (0.5g / mL), the temperature of the reaction system was raised to 80°C, a DMF solution (1.0g / mL) of LBL (35.7mmol) was added dropwise, After the dropwise addition, keep the temperature and continue the reaction for 4.0 h, lower to room temperature, then add DMF to make a solution with a concentration of about 10%, settle with 8 times the volume of glacial ether, and dry the obtained solid under vacuum at 35°C.
[0072] Dissolve the solid in the organic solvent chloroform to make a concentration of 6.0% (g / mL), use a polytetrafluoroethylene film to volatilize at 25°C under normal pressure for 80 hours, remove the film from the film, and dry it in vacuum at room temperature , to obtain the pH-sensitive medical polyurethane urea film material I, and prepare ...
Embodiment 2
[0074] Under the protection of dry nitrogen, 8.950g (50mmol) terminal dihydroxypyridine compound (VP-(OH) 2 ) with 5.0g (5mmol) polyethylene glycol (PEG, M n =1000) were mixed, N,N-dimethylformamide (DMF) was added to dissolve (0.5g / mL), the temperature of the reaction system was raised to 85°C, a DMF solution (1.0g / mL) of LBL (56.1mmol) was added dropwise, After the dropwise addition, keep the temperature and continue the reaction for 3.5 h, lower to room temperature, then add DMF to make a solution with a concentration of about 10%, settle with 8 times the volume of glacial ether, and dry the obtained solid under vacuum at 35°C.
[0075] Dissolve the solid in the organic solvent chloroform to make a concentration of 5.5% (g / mL), use a polytetrafluoroethylene film to volatilize at 25°C under normal pressure for 80 hours, remove the film from the film, and dry it in vacuum at normal temperature , to obtain pH-sensitive medical polyurethane urea membrane material II, and prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com