Branched comb polyaromatic ether sulfone as well as preparation method and application thereof
A polyarylethersulfone and comb-type technology, which is applied in the field of anion exchange membrane fuel cells, can solve problems such as no reports, and achieve the effects of easy preparation, excellent alkali stability and mechanical properties, and high performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
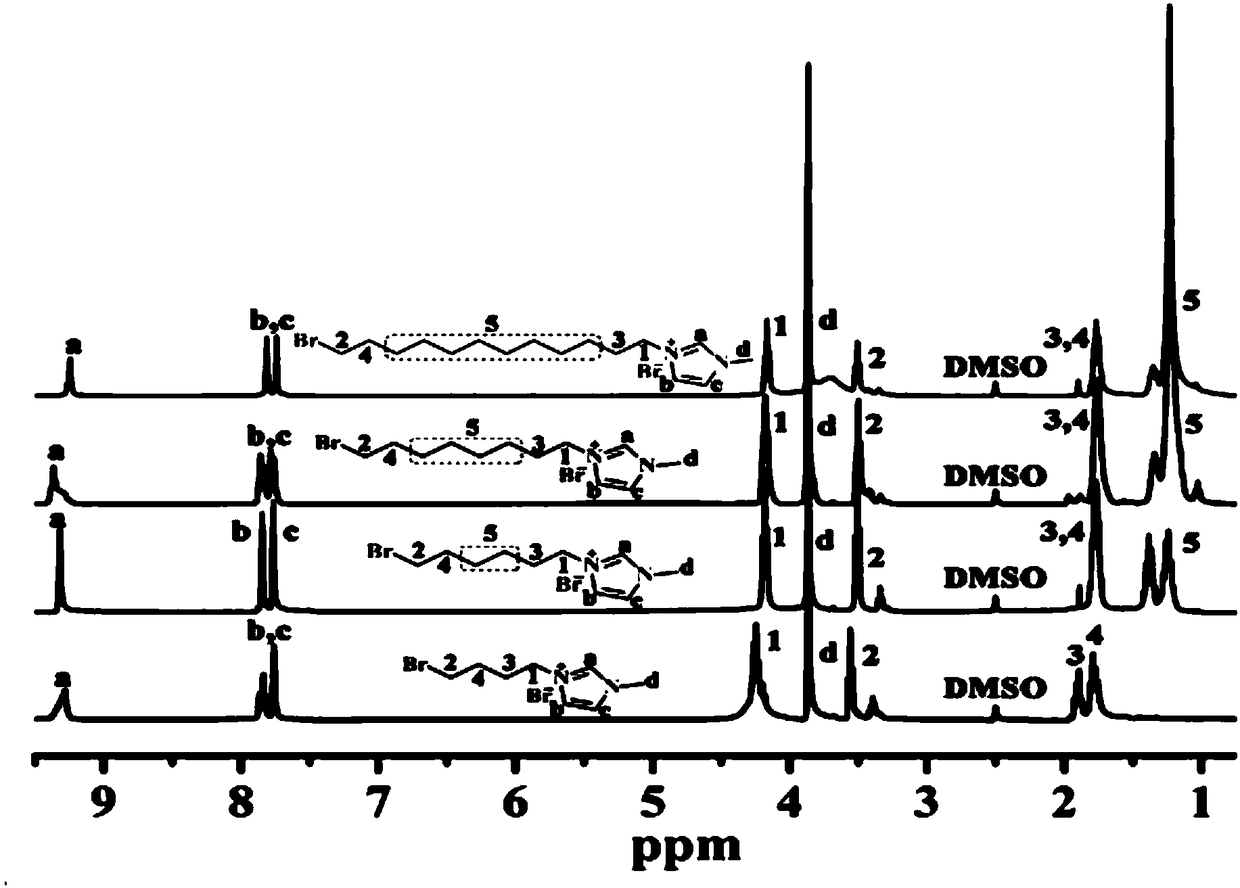
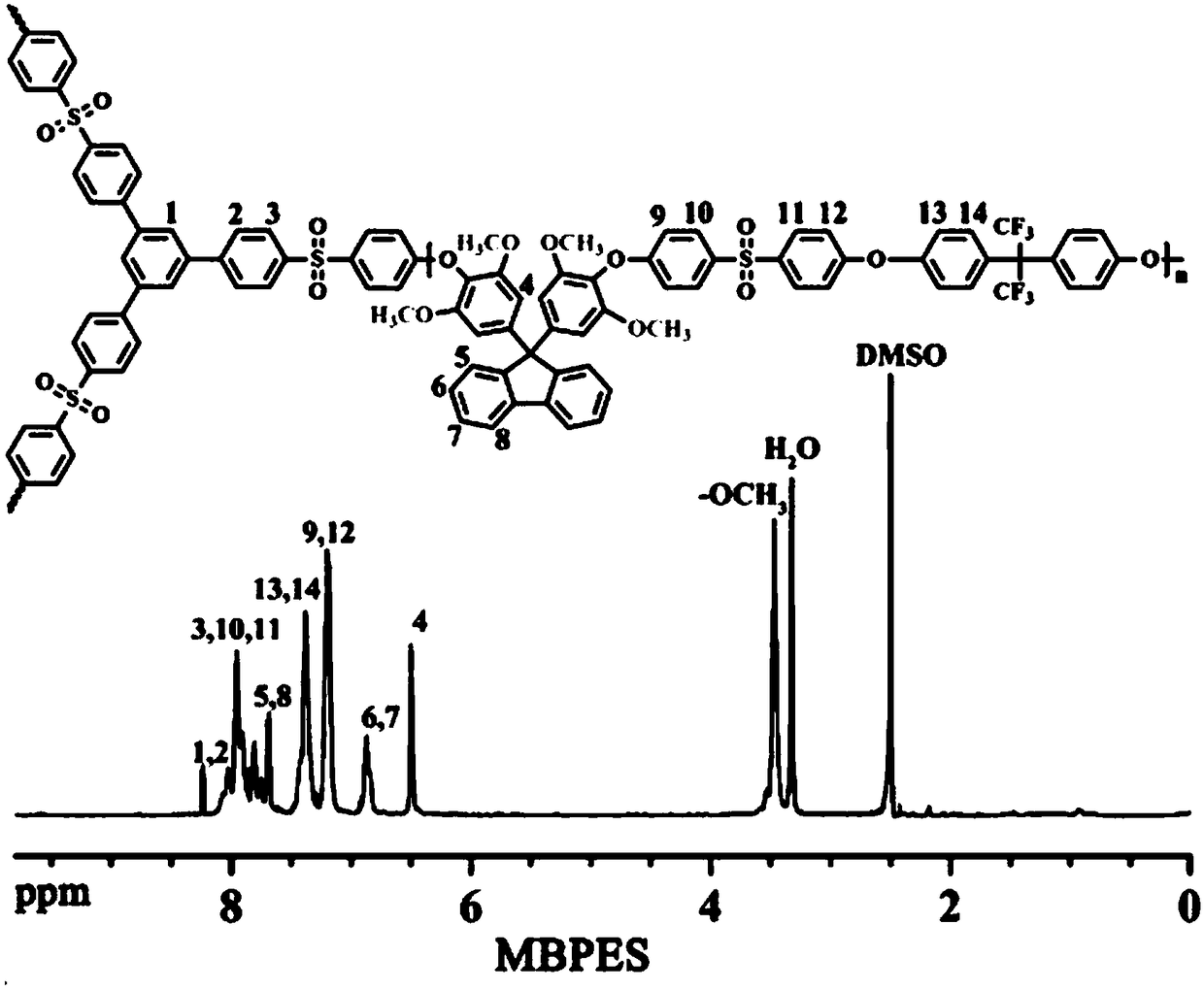
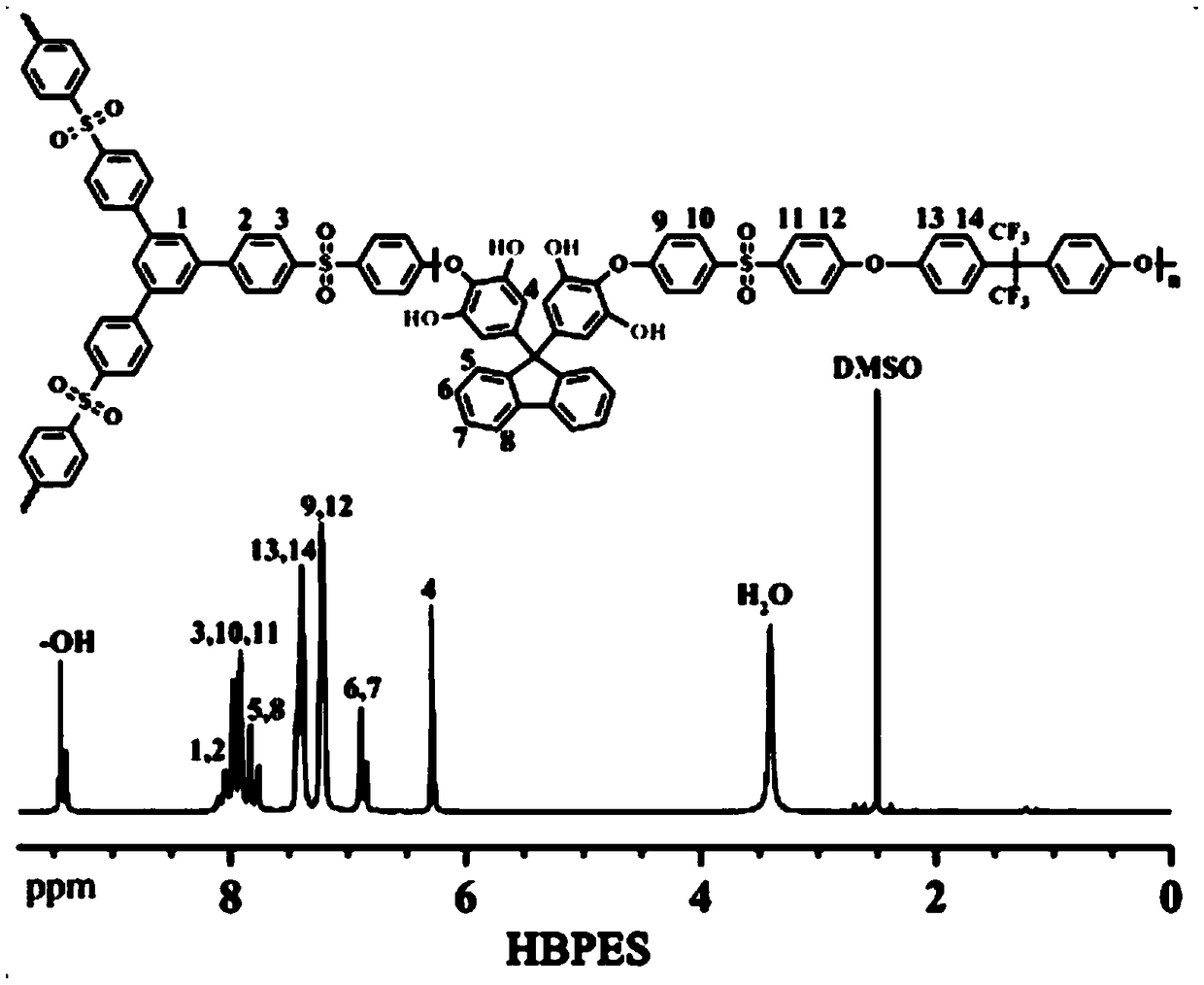
[0045] The embodiment of the present invention provides a method for preparing the branched comb-type polyarylethersulfone as described above, comprising: combining the hydroxyl-containing branched polyarylethersulfone represented by formula III with the bromoalkylimidazole represented by formula IV Onium salt is reacted to obtain the branched comb polyaryl ether sulfone shown in formula I; wherein, n is selected from an integer of 1 to 400; x is selected from an integer of 7 to 16;
[0046]
[0047] The method provided in the embodiment of the present invention prepares a high-performance branched comb-shaped polymer anion exchange membrane material, and the method is simple and easy, and the cost is low.
Embodiment 1
[0063] The synthesis of embodiment 1 imidazolium bromide
[0064] As shown in Formula B, add 80g of 1,8-dibromooctane into a three-necked flask containing a condensing reflux device and a constant-pressure dropping funnel, add 4g of 1-methylimidazole and 50mL of acetone into the constant-pressure dropping funnel, and slowly Add it dropwise into the three-necked flask and stir evenly. The reaction temperature is 40° C., and the reaction is carried out in a nitrogen atmosphere for 15 hours. When the reaction was terminated, the solution after the reaction was filtered, the filtrate was taken, and the acetone solution with a low boiling point was removed by rotary evaporation of the filtrate, then the light yellow liquid after rotary evaporation was poured into a 500mL extraction bottle, and extracted with ethyl acetate and ether solvent for 3 The second time, take the light yellow oily liquid and place it in a vacuum oven at 50° C. for drying for 24 hours to obtain the bromoalky...
Embodiment 2
[0066] According to the method of Example 1, the bromoalkylimidazolium salt shown in formula IV was prepared, wherein, x was 12, which was denoted as Br-12-Im; the yield was 73%.
[0067] In addition, according to the method of Example 1, bromoalkylimidazolium salts shown in formula IV were respectively prepared, wherein, x is 4, denoted as Br-4-Im; x is 6, denoted as Br-6-Im .
[0068] H NMR spectroscopy was used to characterize the structures of the prepared bromoalkylimidazolium salts with different chain lengths. For the results, see figure 1 . figure 1 Among them, the characteristic peaks of the corresponding hydrogen atoms on the monomer are reflected in the spectrograms, the proton peaks on the imidazole ring appear at 9.27, 7.83 and 7.85ppm, the methyl proton peaks on the imidazole ring appear at 3.86ppm, and at 1- 2ppm corresponds to the proton peak on the alkyl chain. Comparing imidazolium salt monomers with different chain lengths, it is found that the area corre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Young's modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com