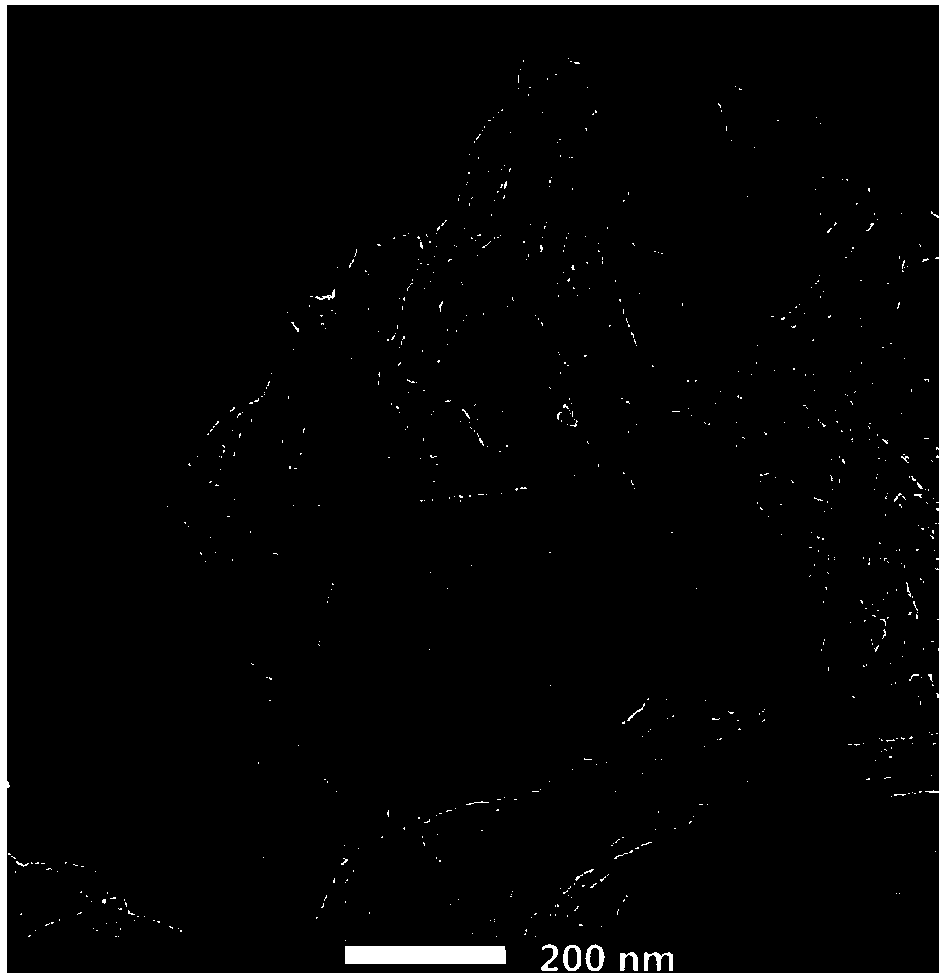
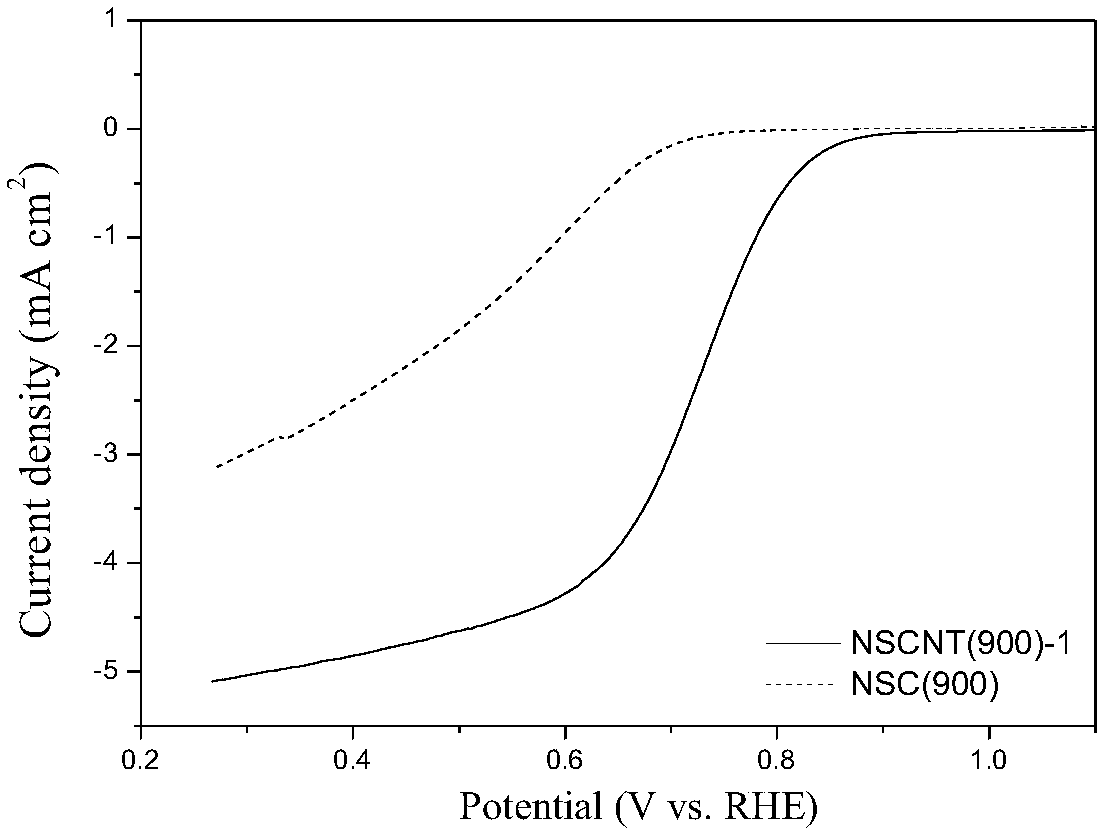
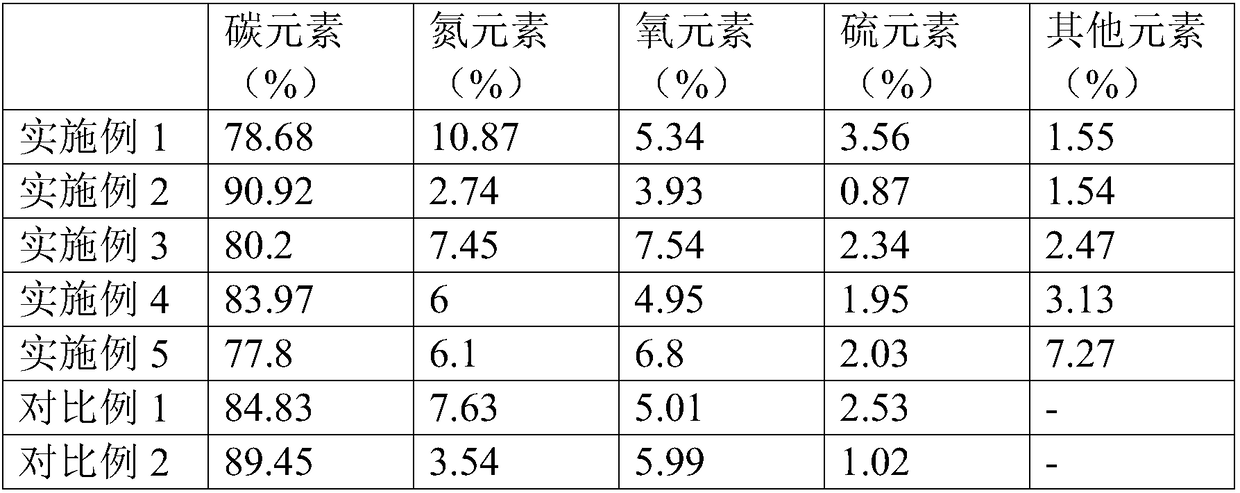
Preparation method and application of nitrogen-sulfur co-doped carbon nanotube
A technology of nitrogen-sulfur co-doping and carbon nanotubes, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problem of low preparation efficiency, harsh conditions, and nitrogen-sulfur co-doping nanomaterials Complicated process and other issues, to achieve large specific surface area, low cost, and improve electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation process of the nitrogen and sulfur co-doped carbon nanotubes of the present invention is specifically as follows:
[0024] (1) Modified halloysite: Disperse pure halloysite in 0.1-0.5 mol / L hydrochloric acid and continue mechanical stirring for 8-24 hours to obtain acidified modified halloysite.
[0025] (2) Synthesis of halloysite / polythiophene composite material: Dissolve modified halloysite and thiophene in an organic solvent at the same time (the mass ratio of thiophene monomer to halloysite is 0.5-2:1; halloysite and organic solvent The mass ratio of 0.05~0.15:1), keep the magnetic stirring for 20~40min, then add the oxidant anhydrous ferric chloride to the system (the mass ratio of anhydrous ferric chloride to thiophene is 3~5:1), 0 Continue to stir and react for 6-12 hours at ~5°C, filter, wash with absolute ethanol, and dry to obtain halloysite / thiophene composite material;
[0026] (3) Synthesize halloysite / polythiophene / polypyrrole composite material...
Embodiment 1
[0033] (1) Modified halloysite: Disperse pure halloysite in 0.2mol / L hydrochloric acid and continue mechanical stirring for 16 hours to obtain acidified modified halloysite.
[0034] (2) Synthesis of halloysite / polythiophene composite materials: 3g modified halloysite and 0.5g thiophene were simultaneously dissolved in 20mL chloroform (the mass ratio of thiophene monomer to halloysite was 0.5:1; halloysite and organic The mass ratio of solvent is 0.15:1), keep magnetic stirring for 20min, then add 2g of oxidant anhydrous ferric chloride (the mass ratio of anhydrous ferric chloride to thiophene is 4:1), continue stirring at 5℃ React for 12h, filter, wash with absolute ethanol, and dry to obtain halloysite / polythiophene composite material;
[0035] (3) Synthesize halloysite / polythiophene / polypyrrole composite material: mix 1g of composite material in (2) with 20mL deionized water (the mass ratio of composite material and deionized water is 0.05:1), ultrasonically disperse for 30 minu...
Embodiment 2
[0039] (1) Modified halloysite: Disperse pure halloysite in 0.1 mol / L hydrochloric acid and continue mechanical stirring for 12 hours to obtain acidified modified halloysite.
[0040] (2) Synthesis of halloysite / polythiophene composite material: 3g modified halloysite and 3g thiophene are simultaneously dissolved in 60mL toluene (the mass ratio of thiophene monomer to halloysite is 1:1; halloysite and organic solvent The mass ratio of 0.05:1), keep magnetic stirring for 30min, then add 9g of oxidizing agent anhydrous ferric chloride (the mass ratio of anhydrous ferric chloride to thiophene is 3:1), continue to stir the reaction at 0℃ 9h, filter, wash with absolute ethanol, and dry to obtain halloysite / polythiophene composite;
[0041] (3) Synthesize halloysite / polythiophene / polypyrrole composite material: mix 1g of the composite material in (2) with 12.5mL deionized water (the mass ratio of composite material to deionized water is 0.08:1), ultrasonically disperse for 20 minutes, A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com