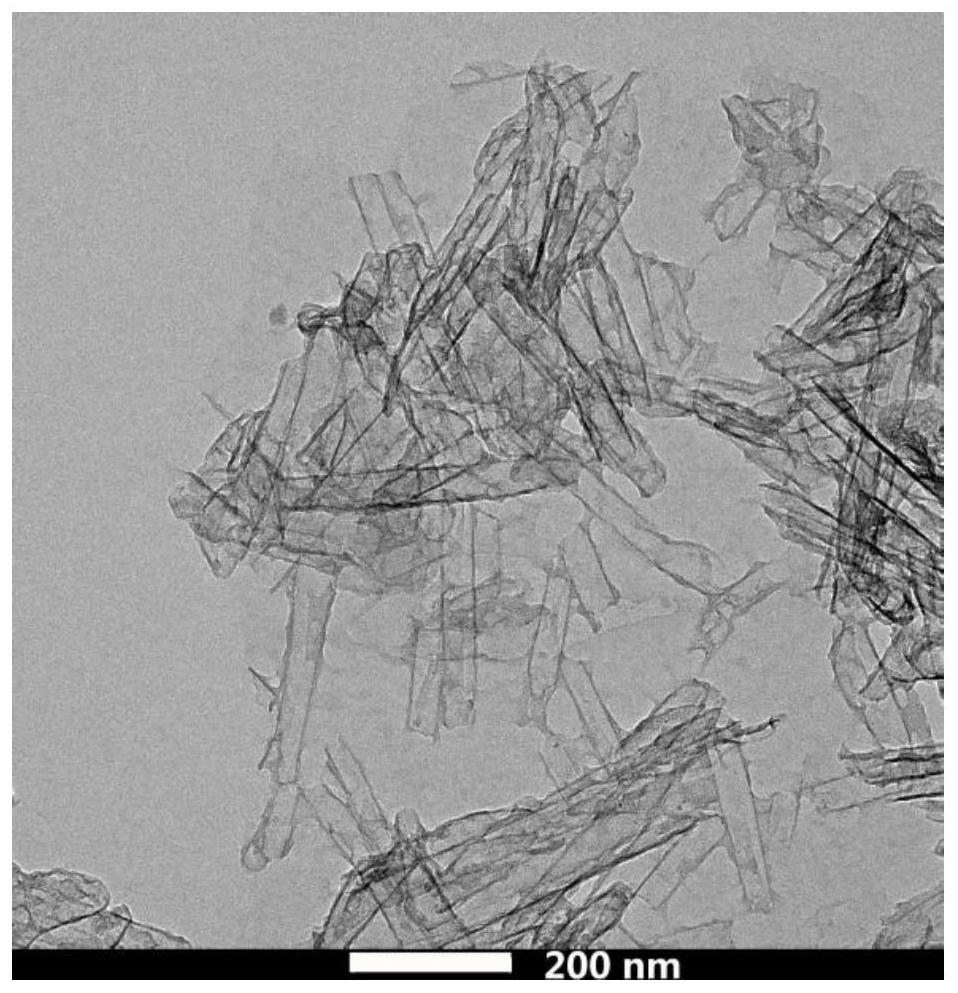
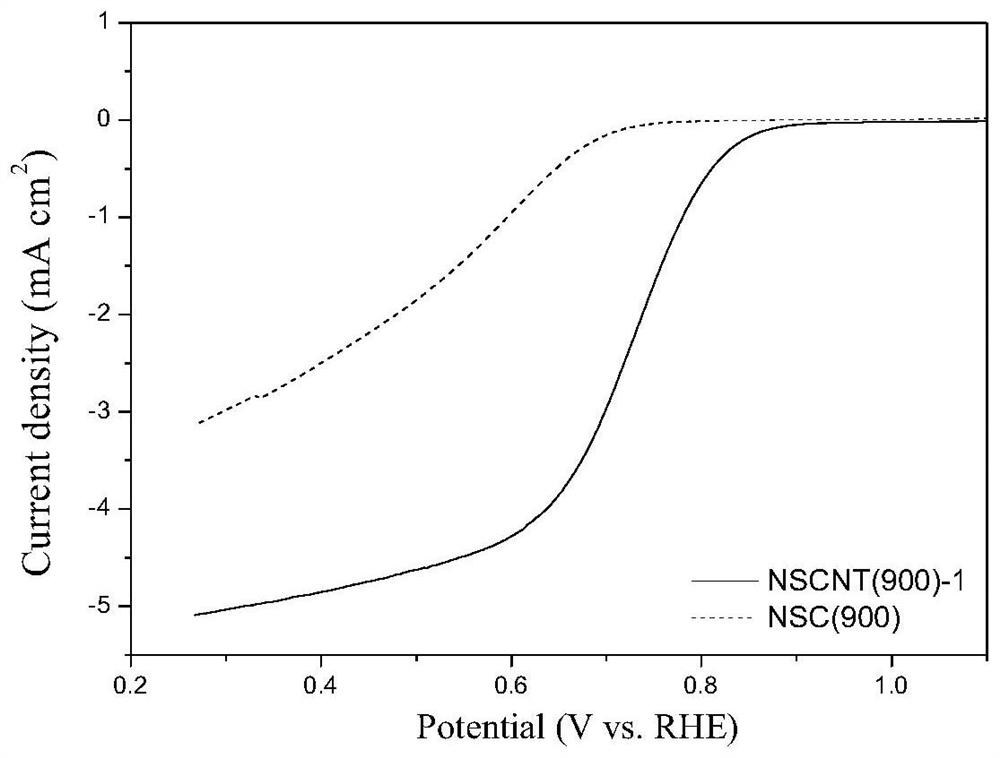
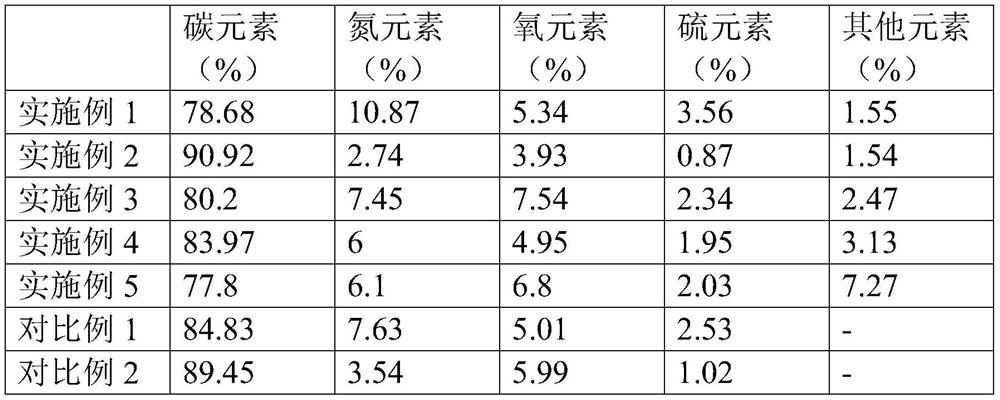
Preparation method and application of nitrogen and sulfur co-doped carbon nanotubes
A nitrogen-sulfur co-doping, carbon nanotube technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc. problems such as low efficiency, to achieve the effect of improving electrochemical performance, low cost, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation process of the nitrogen-sulfur co-doped carbon nanotube of the present invention is specifically as follows:
[0024] (1) Modified halloysite: disperse pure halloysite in 0.1-0.5 mol / L hydrochloric acid, and continue mechanical stirring for 8-24 hours to obtain acidified modified halloysite.
[0025] (2) Synthesis of halloysite / polythiophene composite material: Dissolve modified halloysite and thiophene in an organic solvent at the same time (the mass ratio of thiophene monomer to halloysite is 0.5-2:1; halloysite and organic solvent Mass ratio of 0.05~0.15:1), keep magnetic stirring for 20~40min, then add oxidant anhydrous ferric chloride to the system (mass ratio of anhydrous ferric chloride to thiophene is 3~5:1), 0 Continue to stir and react at ~5°C for 6-12 hours, filter, wash with absolute ethanol, and dry to obtain the halloysite / thiophene composite material;
[0026] (3) Synthesis of halloysite / polythiophene / polypyrrole composite material: Mix a...
Embodiment 1
[0033] (1) Modified halloysite: disperse pure halloysite in 0.2 mol / L hydrochloric acid, and continue mechanical stirring for 16 hours to obtain acidified modified halloysite.
[0034](2) Synthesis of halloysite / polythiophene composite material: 3 g of modified halloysite and 0.5 g of thiophene were simultaneously dissolved in 20 mL of chloroform (the mass ratio of thiophene monomer to halloysite was 0.5:1; halloysite and organic The mass ratio of the solvent is 0.15:1), keep magnetic stirring for 20min, then add 2g oxidant anhydrous ferric chloride to the system (the mass ratio of anhydrous ferric chloride to thiophene is 4:1), and continue stirring at 5°C React for 12 hours, filter, wash with absolute ethanol, and dry to obtain the halloysite / polythiophene composite material;
[0035] (3) Synthesis of halloysite / polythiophene / polypyrrole composite material: Mix 1 g of the composite material in (2) with 20 mL of deionized water (the mass ratio of composite material to deioniz...
Embodiment 2
[0039] (1) Modified halloysite: disperse pure halloysite in 0.1 mol / L hydrochloric acid, and continue mechanical stirring for 12 hours to obtain acidified modified halloysite.
[0040] (2) Synthesis of halloysite / polythiophene composite material: 3g modified halloysite and 3g thiophene were simultaneously dissolved in 60mL toluene (the mass ratio of thiophene monomer to halloysite was 1:1; halloysite and organic solvent The mass ratio of ferric chloride to thiophene is 0.05:1), keep magnetic stirring for 30min, then add 9g oxidant anhydrous ferric chloride to the system (the mass ratio of anhydrous ferric chloride to thiophene is 3:1), and continue to stir the reaction at 0°C 9h, filter, wash with absolute ethanol, and dry to obtain the halloysite / polythiophene composite material;
[0041] (3) Synthesis of halloysite / polythiophene / polypyrrole composite material: Mix 1 g of the composite material in (2) with 12.5 mL of deionized water (mass ratio of composite material to deioni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com