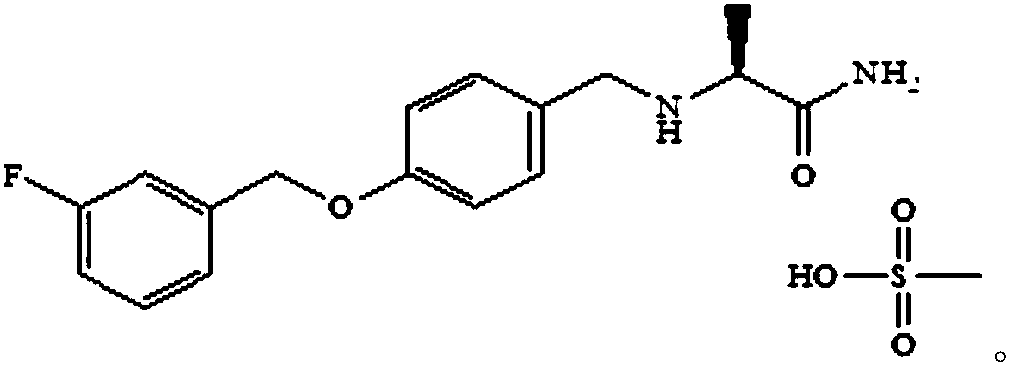
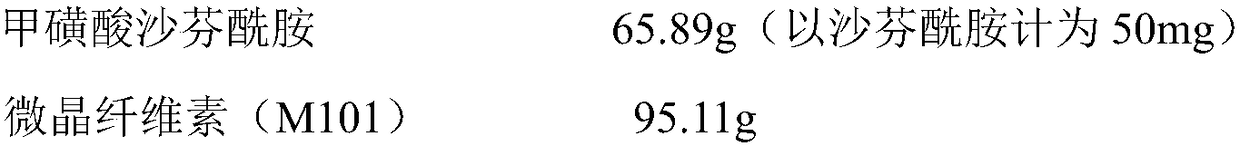Safinamide mesylate tablet and preparation method thereof
A safinamide mesylate tablet and prescription technology, which is applied in the field of medicine, can solve problems such as inconsistency with the mechanism of action, toxic impurities, and poor powder fluidity, and achieve simple and easy access to excipients, improved dissolution rate, and low requirements for production equipment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The preparation of embodiment 1 tablet
[0015]
[0016]
[0017] (1) Weighing the ingredients: Weigh the required raw and auxiliary materials according to the prescription amount, and if there are agglomerates, the raw and auxiliary materials are crushed and sieved through 80 sieves;
[0018] (2) Granulation and granulation: Take the prescribed amount of safinamide mesylate, microcrystalline cellulose, hypromellose and crospovidone (internal addition) and put it in a wet mixing granulator (G20 type) , set stirring at 330rpm, chopping at 2500rpm, stirring and mixing for 240s to obtain a dry-mixed material; set stirring at 220rpm, chopping at 1500rpm, add wetting agent while stirring, finish adding within 30s, and then discharge after stirring for 60s. The material is placed in a GZX-9240MBE electric blast drying oven, the drying temperature is set to 60°C, and the drying is carried out until the moisture content is ≤2.0%, and the material is discharged, and the dr...
Embodiment 2
[0021] The preparation of embodiment 2 tablet
[0022]
[0023]
[0024] (1) Weighing the ingredients: Weigh the required raw and auxiliary materials according to the prescription amount, and if there are agglomerates, the raw and auxiliary materials are crushed and sieved through 80 sieves;
[0025](2) Granulation and granulation: Take the prescribed amount of safinamide mesylate, microcrystalline cellulose, hypromellose and crospovidone (internal addition) and put it in a wet mixing granulator (G20 type) , set stirring at 330rpm, chopping at 2500rpm, stirring and mixing for 240s to obtain a dry-mixed material; set stirring at 220rpm, chopping at 1500rpm, add wetting agent while stirring, finish adding within 30s, and then discharge after stirring for 60s. The material is placed in a GZX-9240MBE electric blast drying oven, the drying temperature is set to 60°C, and the drying is carried out until the moisture content is ≤2.0%, and the material is discharged, and the dry...
Embodiment 3
[0028] Embodiment 3 Dissolution Comparison
[0029] Refer to the "Technical Guidelines for Dissolution Test of Ordinary Oral Solid Preparations" and the relevant guidelines in the second appendix of the 2010 edition of the Chinese Pharmacopoeia. Dissolution measurement method adopts paddle method, with pH1.0 hydrochloric acid solution 1000ml as standard dissolution medium, rotating speed is 50rpm, under this rotating speed condition, embodiment sample and original research sample are in pH6.8 phosphate buffered saline buffer solution, water, pH1.0 In the four dissolution media of hydrochloric acid solution and pH4.5 acetate buffer, the dissolution behavior was similar.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com