Water-soluble aza-alpha-naphthoflavone compound and preparation method and medical application thereof
A water-soluble, naphthoflavone technology, applied in pharmaceutical formulations, organic chemistry, anti-tumor drugs, etc., can solve the problems of decreased selectivity, decreased inhibitory activity, etc., and achieves the effects of simple operation, easy availability of raw materials, and good water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
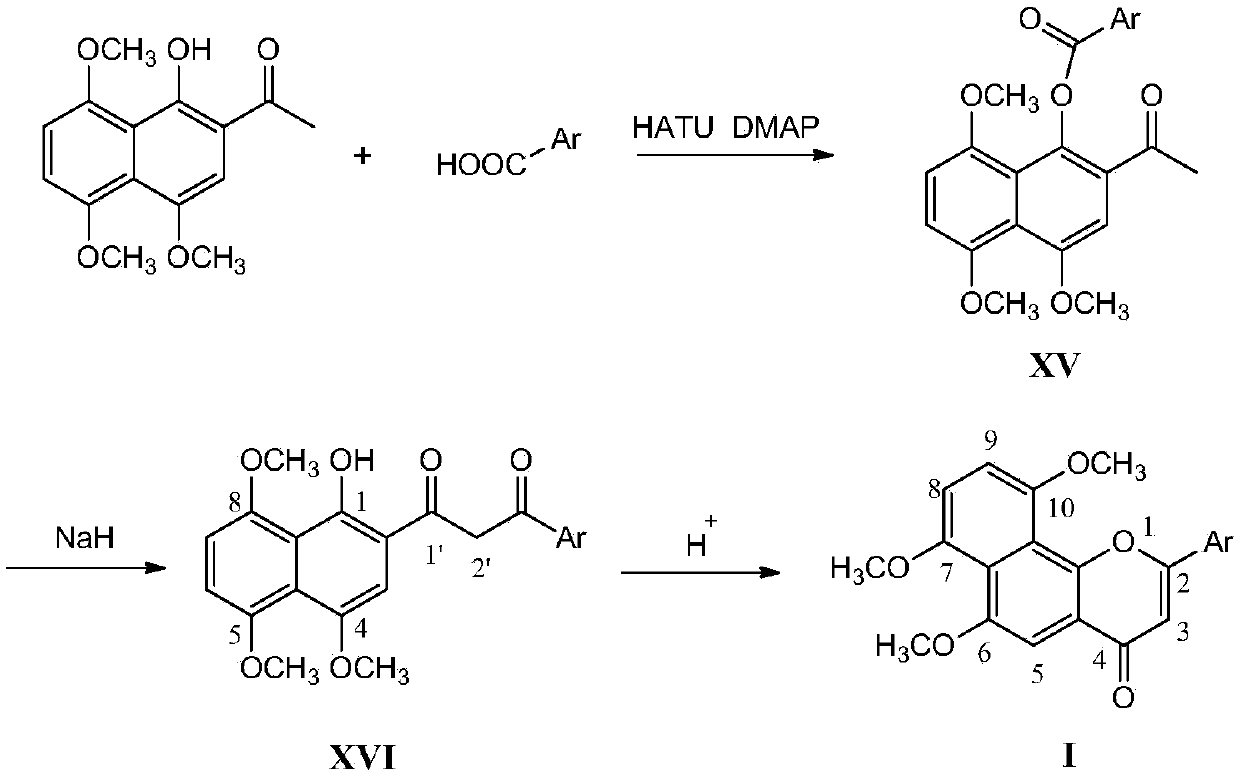
[0044] This example relates to a 6,7,10-trimethoxy-2-(2'-pyridyl)naphtho[1,2-b]pyran-4-one (Ⅲ-1 ) preparation method, such as figure 1 shown, including the following steps:
[0045]
[0046] Step 1, dissolve 2-acetyl-4,5,8-trimethoxy-1-naphthol in anhydrous N,N'-dimethylformamide, add 3.0 equivalents of 2-pyridinecarboxylic acid, add 3.0 times the equivalent of 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) and catalytic amount of 4-dimethylaminopyridine (DMAP ), after stirring at room temperature for 12h, adding saturated ammonium chloride solution to quench the reaction, CH 2 Cl 2 Extract and combine the organic layers. The organic layer was washed with saturated ammonium chloride solution, dried over anhydrous sodium sulfate, concentrated, and silica gel column chromatography to obtain 4,5,8-trimethoxy-2-acetyl-1-naphthol-2'-pyridinecarboxylic acid The ester is a yellow solid, and the yield is 92%.
[0047] Step 2: Dissolve 4,5,8...
Embodiment 2
[0050] This example relates to a 6,7,10-trimethoxy-2-(3'-fluoro-2'-pyridyl)naphtho[1,2-b]pyran-4- The preparation method of ketone (Ⅲ-2), such as figure 1 shown, including the following steps:
[0051]
[0052] The steps of this example are the same as the steps of Example 1, in Step 1, 2-pyridinecarboxylic acid is replaced by 6-fluoro-2-pyridinecarboxylic acid. The product is yellow powder, and the total yield is 20%. 1 H NMR (400MHz, DMSO-d 6 ):δ8.43(d,J=7.7Hz,1H),8.32–8.25(m,1H),7.48(d,J=7.6Hz,1H),7.36–7.24(m,3H),7.17(s, 1H), 4.14(s,3H), 3.95(s,3H), 3.86(s,3H).
Embodiment 3
[0054] This example relates to a 6,7,10-trimethoxy-2-(3'-chloro-2'-pyridyl)naphtho[1,2-b]pyran-4- The preparation method of ketone (Ⅲ-3), such as figure 1 shown, including the following steps:
[0055]
[0056] The steps of this example are the same as the steps of Example 1, in Step 1, 2-pyridinecarboxylic acid is replaced by 6-chloro-2-pyridinecarboxylic acid. The product was light yellow powder, and the total yield was 26%. 1 H NMR (400MHz, DMSO-d 6 ):δ8.26(d,J=7.0Hz,2H),7.74(d,J=7.0Hz,1H),7.27(s,3H),7.16(s,1H),4.09(s,3H),3.91 (s,1H),3.82(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com