A high-strength injectable intraocular lens material and its preparation method and application
An intraocular lens, high-strength technology, applied in the field of biomedicine, can solve the problems of unavoidable cataract surgery complications, poor physical and mechanical strength of human lens, etc., achieve controllable drug loading rate, reduce non-specific drug release behavior, reduce the damaging effects of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This example provides the preparation of the intraocular lens material of the present invention.
[0035] 1 Preparation of prodrug monomer
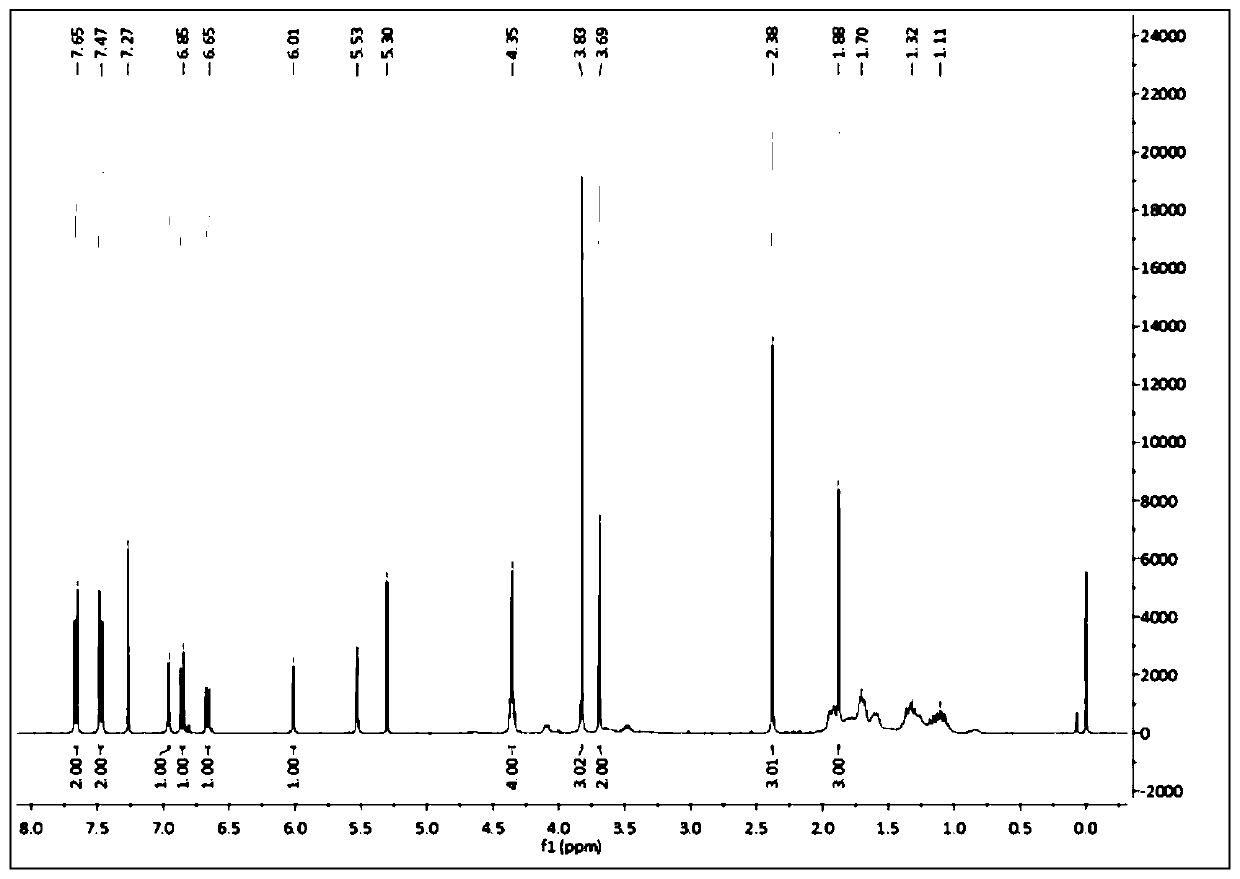
[0036] Dissolve 130.10 mg of anhydrous hydroxyethyl methacrylate and 357.79 mg of indomethacin in 20 ml of anhydrous dichloromethane under stirring conditions to obtain a solution; add N,N'-dicyclohexylcarbodi 412.66 mg of imine and 244.32 mg of 4-dimethylaminopyridine were stirred and reacted at room temperature for 24 hours; the reaction was detected by TCL, and after the reaction was complete, the reaction solution was washed with water, dried and filtered, and rotary distilled; the resulting product was redissolved in a small amount of di Chloromethane, purified by silica gel chromatography, and then converted to indomethacin-HEMA prodrug monomer after rotary distillation. Obtain prodrug monomer 303.56mg, yield is 62.21% The NMR spectrum of indomethacin-HEMA is as attached figure 1 shown.
[0037] 2 select the monomer
[00...
Embodiment 2
[0046] This example provides the preparation of the intraocular lens of the present invention.
[0047] 1 Preparation of prodrug monomer
[0048] Prepared by the method of Example 1, the indomethacin-HEMA prodrug monomer was obtained.
[0049] 2 select the monomer
[0050] The monomers used in this embodiment and their consumption are shown in the table below:
[0051] type name Dosage (wt%) Thermosensitive functional monomer Vinylpyrrolidone 75 Acrylate monomer Methyl methacrylate 15 prodrug monomer Indomethacin-HEMA 7.5 crosslinking agent Butanediol dimethacrylate 0.5 Initiator Dibenzoyl peroxide 2
[0052] 3. Preparation of intraocular lens materials
[0053] The above four monomers and initiators were mixed uniformly under the protection of nitrogen and then poured into a glass mold, heated and polymerized in an oven at 60°C for 18 hours, then the temperature was raised to 90°C, and the reaction was continued for 6...
Embodiment 3
[0056] This example provides the preparation of the intraocular lens of the present invention.
[0057] 1 Preparation of prodrug monomer
[0058] Prepared by the method of Example 1, the indomethacin-HEMA prodrug monomer was obtained.
[0059] 2 select the monomer
[0060] The monomers used in this embodiment and their consumption are shown in the table below:
[0061] type name Dosage (wt%) Thermosensitive functional monomer Dimethylaminoethyl methacrylate 85 Acrylate monomer Methyl methacrylate 5 prodrug monomer Indomethacin-HEMA 7.5 crosslinking agent Hexylene glycol acrylate 0.8 Initiator ammonium peroxodisulfate 1.7
[0062] 3. Preparation of intraocular lens materials
[0063] Dissolve the above four monomers and initiators in 5ml of acetic acid: water = 1:1 under the protection of nitrogen, then inject into the glass mold, heat and polymerize in an oven at 60°C for 18 hours, then raise the temperature to 90°C,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com