Preparation method and application for monoclonal antibody against HPV16 L1 protein
A monoclonal antibody and protein technology, applied in antiviral immunoglobulins, immunoglobulins, chemical instruments and methods, etc., can solve the problem that the HPV virus antigen protein is difficult to apply, the detection technology of HPVL1 protein is lagging behind, and it is difficult to separate natural Virus and other problems, to achieve the effect of stabilizing antibody secretion capacity, extensive research application value and commercial use value, and improving specificity and affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of hybridoma cell lines secreting anti-HPV16 L1 protein monoclonal antibody
[0042] 1. Main Reagents and Materials
[0043]Freund's complete adjuvant, Freund's incomplete adjuvant, HAT, HT, PEG-1500, RPM1-1640 cell culture medium, fetal bovine serum were purchased from Gibco Company, HRP-labeled goat anti-mouse IgG was purchased from Sigma Company, liquid AEC enzyme The substrate kit was purchased from Zhongshan Jinqiao Company, the BCA protein concentration determination kit was purchased from Solarbio Company, the monoclonal antibody subtype determination kit was purchased from Beijing Yiqiao Shenzhou Biotechnology Co., Ltd.; the commercial anti-HPV16 L1 monoclonal antibody was purchased from Abcam (Abcam, UK); plasmids p6SheLL, p16SheLL, p18SheLL, p31SheLL, p45SheLL, p52SheLL and p58SheLL containing the corresponding HPV L1 and L2 ORF region genes were purchased from Addgene, provided by Prof. John Schiller (Addgene, USA), BALB / c Mice were pu...
Embodiment 2
[0068] Example 2: Identification of hybridoma cell lines secreting anti-HPV16 L1 protein monoclonal antibody
[0069] 1. Screening, identification and subcloning of hybridoma cells
[0070] (1) ELISA screening
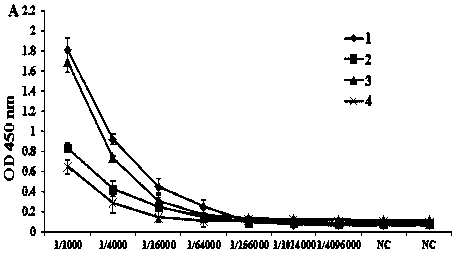
[0071] In the first round of ELISA screening, SUMO1-16 L1 recombinant protein (diluted with CBS 1:100) was used as the coating source, and 22 ELISA plates were coated to screen the hybridoma cell culture supernatant; the 96 strains that were initially screened were hybridized Tumor cell lines were transferred to two 48-well cell plates for further culture.
[0072] In the second round of ELISA screening, SUMO1-16 L1 purified protein (diluted with CBS 1:100) and supernatant of SUMO-tag recombinant protein sonicated (diluted with CBS 1:1000) were selected as the coating source, and SUMO-tag recombinant protein A total of 67 positive hybridoma cell lines were screened out after eliminating false positives.
[0073] In the third round of ELISA screening, the pre-prepare...
Embodiment 3
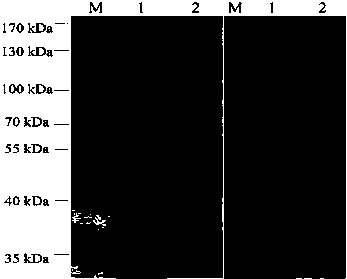
[0083] Example 3: Preparation and purification of anti-HPV16L1 protein monoclonal antibody ascites
[0084] 1. Preparation of anti-HPV16L1 protein monoclonal antibody ascitic fluid
[0085] The monoclonal hybridoma cell line 19H7 in Example 2 was expanded and cultured, and the titer of the culture supernatant was measured by ELISA to ensure the stability of the monoclonal cell line, and the cells were collected for large-scale preparation of cloned antibodies. The specific steps were as follows:
[0086] (1) Multiparous female BALB / c mice were selected, and 500 μl of sterilized paraffin was injected intraperitoneally to stimulate immune cells to promote the proliferation of hybridoma cells.
[0087] (2) Observe the state of the mice, and after 7-10 days, the 7 Inject the amount of cells into the monoclonal positive cells prepared in advance, and observe the state of the mice in time;
[0088] (3) After 10 days, extract ascites, centrifuge at 8000 r / min, 4°C for 20 min to rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com