Semaglutide contained oral prolonged-release preparation and preparation method thereof
A sustained-release preparation, semaglutide technology, applied in medical preparations containing active ingredients, medical preparations with non-active ingredients, pill delivery, etc., can solve the problem of high cost for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
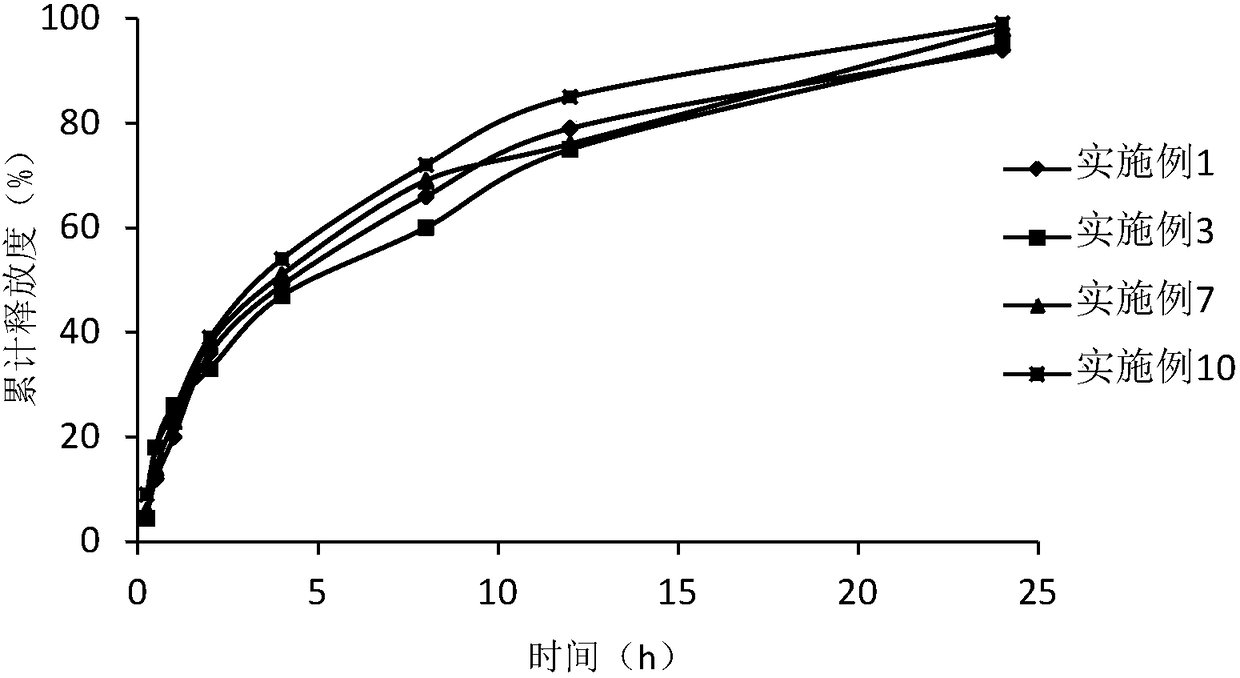
Examples
Embodiment 1
[0032] Prescription for oral sustained-release preparations containing semaglutide (content per tablet)
[0033]
[0034] Pass the raw materials and auxiliary materials through 80-mesh sieves respectively, set aside, and configure the prescription quantity of 1000 samples.
[0035] ① Weigh semaglutide, metformin, cellulose acetate, ethyl cellulose, and polyethylene glycol according to the prescription amount; dissolve polyethylene glycol, semaglutide, and metformin in 1 mL of water as the internal water phase W1 ; Dissolve cellulose acetate in 10mL of dichloromethane as the organic phase O; dissolve polyvinyl alcohol in 50mL of water as the external aqueous phase W2;
[0036] ② Add the internal water phase W1 to the organic phase O, and ultrasonically emulsify with a power of 250W for 10 minutes to form a uniform colostrum W1 / O;
[0037] ③ Add colostrum W1 / O to the external water phase W2, and ultrasonically emulsify with a power of 250W for 10 minutes to form double emuls...
Embodiment 2
[0043] Prescription for oral sustained-release preparations containing semaglutide (content per tablet)
[0044]
[0045] Pass the raw materials and auxiliary materials through 80-mesh sieves respectively, set aside, and configure the prescription quantity of 1000 samples.
[0046] ① Weigh semaglutide, ciglitazone, cellulose acetate, polyethylene, and Tween-80 according to the prescription amount; dissolve Tween-80, semaglutide, and ciglitazone in 2 mL of water, and prepare Inner water phase W1; cellulose acetate and polyethylene were dissolved in 10 mL of dichloromethane as the organic phase O; polyvinyl alcohol was dissolved in 100 mL of water as the outer water phase W2;
[0047] ② Add the internal water phase W1 to the organic phase O, and ultrasonically emulsify with a power of 300W for 8 minutes to form a uniform colostrum W1 / O;
[0048] ③ Add colostrum W1 / O to the external water phase W2, power 300W ultrasonic emulsification for 8 minutes to form double emulsion W1 / ...
Embodiment 3
[0054] Prescription for oral sustained-release preparations containing semaglutide (content per tablet)
[0055]
[0056] Pass the raw materials and auxiliary materials through 80-mesh sieves respectively, set aside, and configure the prescription quantity of 1000 samples.
[0057] ① Weigh semaglutide, pioglitazone, ethyl cellulose, polyethylene, and mannitol respectively according to the prescription quantity; dissolve mannitol, semaglutide, and pioglitazone in 2 mL of water as the inner water phase W1; 1. Dissolve polyethylene in 10mL of dichloromethane as organic phase O; dissolve polyvinyl alcohol in 100mL of water as external water phase W2;
[0058] ②Add the internal water phase W1 into the organic phase O, and ultrasonically emulsify with a power of 350W for 7 minutes to form a uniform colostrum W1 / O;
[0059] ③ Add colostrum W1 / O to the external water phase W2, and ultrasonically emulsify with a power of 350W for 7 minutes to form double emulsion W1 / O / W2;
[0060] ④...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com