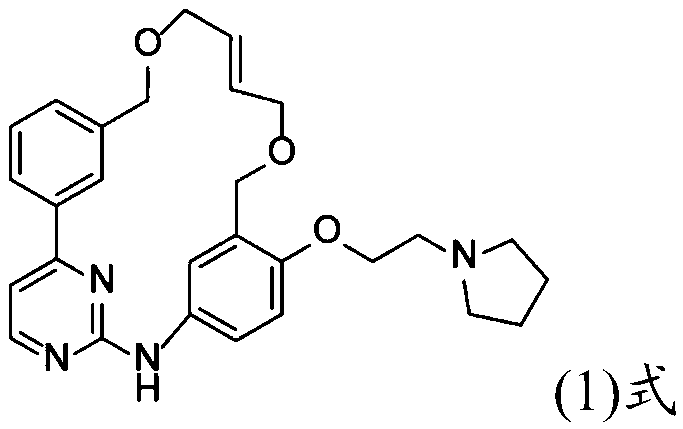
Method for preparing drug intermediate by catalyzing organic silicon supported ion liquid
A technology of ionic liquid and organic silicon, which is applied in the field of medicine and chemical industry, can solve the problems of low production efficiency, long reaction time, low yield, etc., and achieve the effect of realizing recycling and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] One, prepare organosilicon-loaded alkaline ionic liquid as follows:
[0039] 1) Ionic liquid preparation process:
[0040] Mix 40mmol of triethylenediamine and 20mmol of 1,4-dichlorobutane, stir at 90-100°C for 3-5h under nitrogen atmosphere, then add 300ml of n-heptane for ultrasonic dispersion for 20-30min, filter, and reduce at 40°C Pressing and drying to obtain the ionic liquid precursor;
[0041] Dissolve 19.8mmol of imidazole in 100ml of anhydrous methanol solution of 0.2mol / L sodium hydroxide at 40-45°C and stir to dissolve for 10-20min, then add 3.51g of ionic liquid precursor (about 19.8mmol by silver nitrate titration). Chloride ion) stirred at 40-45°C for 6-8h, cooled to room temperature and filtered to remove insoluble sodium chloride, and the filtrate was concentrated to obtain an ionic liquid;
[0042] 2) Loading of ionic liquid
[0043] Dissolve 100g of isopropyl orthosilicate in 100ml of isopropanol and heat to 70-80°C, then add 15g of ionic liquid an...
Embodiment 2
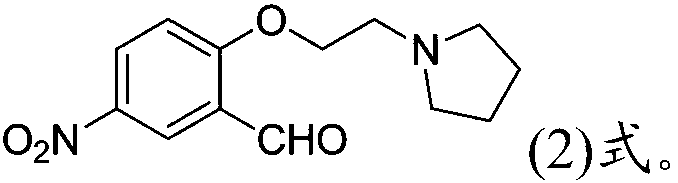
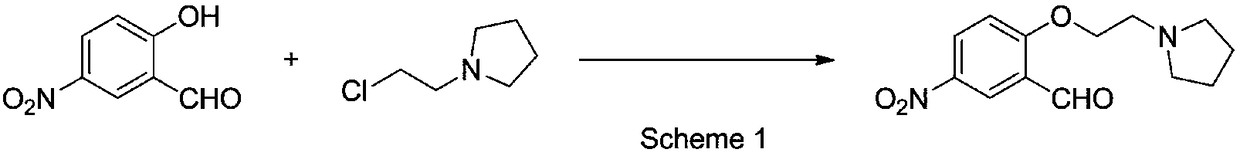
[0052] Lithium iodide was selected as metal iodide to prepare organosilicon-supported alkaline ionic liquid as catalyst to catalyze the condensation reaction of 5-nitrosalicylaldehyde and N-(2-chloroethyl)pyrrolidine to prepare 5-nitro -2-[2-(pyrrolidin-1-yl)ethoxyl]benzaldehyde, the present invention further optimizes the solvent type in this catalytic reaction, the method is as follows:
[0053] Add 5-nitrosalicylaldehyde (1.67g, 10mmol), N-(2-chloroethyl)pyrrolidine (1.74g, 13mmol, 1.3eq) and lithium iodide modified organosilicon support to the parallel synthesizer. Loaded alkaline ionic liquid catalyst (0.5g, ~30%wt), 30ml solvent, react at 80°C (solvent with boiling point lower than 80°C, react at reflux temperature), HPLC detects 5-nitrowater in the reaction solution After the concentration of salicylaldehyde no longer changes within 2h, the area percentage of the substrate 5-nitrosalicylaldehyde, the target product 5-nitro-2-[2-(pyrrolidin-1-yl) in the reaction solution...
Embodiment 3
[0058] On the basis of selecting lithium iodide as metal iodide to prepare organosilicon-loaded alkaline ionic liquid as catalyst and acetonitrile as solvent, the present invention has the following requirements for catalyst consumption, N-(2-chloroethyl)pyrrolidine molar consumption (Based on the molar dosage of 5-nitrosalicylaldehyde) further optimization:
[0059] Organosilicon prepared by adding 5-nitrosalicylaldehyde (1.67g, 10mmol), N-(2-chloroethyl)pyrrolidine (11-15mmol, 1.1-1.5eq), and lithium iodide to a parallel synthesizer Loaded alkaline ionic liquid catalyst (0.17-1.67g, 10-100%wt), 30ml of acetonitrile, reacted at 80°C, after the HPLC detection of the concentration of 5-nitrosalicylaldehyde in the reaction solution no longer changed within 2h, The area percentage of substrate 5-nitrosalicylaldehyde, the area percentage of target product 5-nitro-2-[2-(pyrrolidin-1-yl) ethoxyl] benzaldehyde and Its impurity content (using the area normalization method to carry ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com