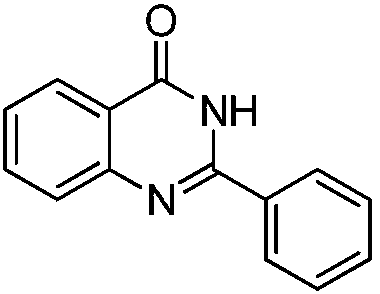
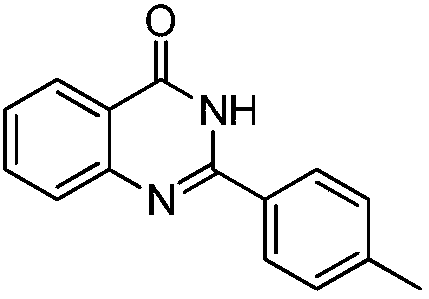
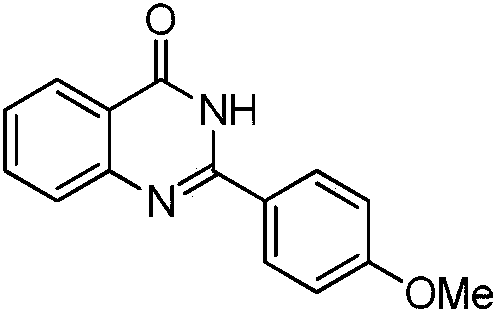
Iron-based catalyst for synthesizing compound with quinazolinone structure, and preparation method and application of iron-based catalyst
An iron-based catalyst, quinazolinone technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems affecting product quality, catalysts cannot be recycled, and product separation is complicated, etc. Achieving the effects of wide substrate applicability, good catalyst stability and high product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 1g of anhydrous ferric chloride and 0.9g of 1-ethyl-3-methylimidazolium chloride salt into acetone, stir at room temperature for 3 hours to obtain a mixed solution; then add 10g of sucrose to the above mixed solution, and Stir at low temperature for 12 hours, then spin evaporate the acetone, dry the obtained solid under vacuum at 60°C for 12 hours, heat it to the target temperature of 600°C in an inert atmosphere, pyrolyze it at a constant temperature for 2 hours, and then cool it down to room temperature naturally to obtain a black powder that is ready for use. Iron-based catalysts for the synthesis of quinazolinone compounds.
Embodiment 2
[0033] Weigh 1g of anhydrous ferric chloride and 0.9g of 1-ethyl-3-methylimidazolium chloride salt into acetone, stir at room temperature for 3 hours to obtain a mixed solution; then add 10g of sucrose to the above mixed solution, and Stir at low temperature for 12 hours, then spin evaporate the acetone, and dry the obtained solid under vacuum at 60°C for 12 hours. After heating to the target temperature of 700°C in an inert atmosphere, pyrolyze at constant temperature for 2 hours, and then cool naturally to room temperature to obtain a black powder that is ready for use. Iron-based catalysts for the synthesis of quinazolinone compounds.
Embodiment 3
[0035] Weigh 1g of anhydrous ferric chloride and 0.9g of 1-ethyl-3-methylimidazolium chloride salt into acetone, stir at room temperature for 3 hours to obtain a mixed solution; then add 10g of sucrose to the above mixed solution, and Stir under low temperature for 12 hours, then spin evaporate the acetone, and dry the obtained solid under vacuum at 60°C for 12 hours, heat it to the target temperature of 800°C in an inert atmosphere, pyrolyze it at a constant temperature for 2 hours, and then cool it down to room temperature naturally to obtain a black powder that is ready for use. Iron-based catalysts for the synthesis of quinazolinone compounds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com