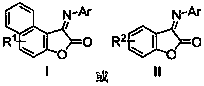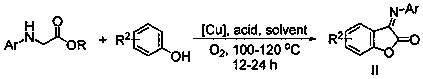Benzofuranone/ naphthofuranone aryl imine compound and synthetic method thereof
A technology of furanone aryl imine and synthesis method, which is applied in the field of benzo/naphthofuranone aryl imine compounds and their synthesis, can solve the problems of harsh reaction conditions and unproposed applicability, and achieve functional group compatibility Strong, easy to separate, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0028] (Z)-1-(4-methylphenylimine)naphtho[2,1-b]furan-2-one
[0029] Add 0.01mmol copper acetate, 0.3mmol acetic acid, 0.15mmol 2-naphthol, 0.1mmol N-(4-methylphenyl)glycine ethyl ester, solvent 1,2-dichloroethane into a 25mL oxygen-filled reactor 2.0mL. Heat to 120°C, keep stirring for 24h, stop the reaction, cool to room temperature, wash with saturated sodium chloride solution (25°C), extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography (washing Removal agent: petroleum ether / dichloromethane) to obtain the target product with a yield of 75%. 1 H NMR (400M, CDCl 3 ): δ8.96(d, J=8.4Hz, 1H), 8.06(d, J=8.8Hz, 1H), 7.88(d, J=8.0Hz, 1H), 7.68(t, J=7.6Hz, 1H ), 7.54-7.51 (m, 1H), 7.29 (d, J=8.8Hz, 1H), 7.26-7.22 (m, 2H). 7.09-7.07 (m, 2H), 2.40 (s, 3H).
Synthetic example 2
[0031] (Z)-1-(4-tert-butylphenylimine)naphtho[2,1-b]furan-2-one
[0032]Add 0.01mmol copper acetate, 0.3mmol acetic acid, 0.15mmol 2-naphthol, 0.1mmol N-(4-tert-butylphenyl) glycine ethyl ester, solvent 1,2-dichloroethyl Alkanes 2.0mL. Heat to 120°C, keep stirring for 24h, stop the reaction, cool to room temperature, wash with saturated sodium chloride solution (25°C), extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography (washing Removal agent: petroleum ether / dichloromethane) to obtain the target product with a yield of 82%. 1 H NMR (400M, CDCl 3 ): δ8.94(d, J=8.4Hz, 1H), 8.03(d, J=8.8Hz, 1H), 7.86(d, J=8.0Hz, 1H), 7.66(t, J=7.6Hz, 1H ),7.50(t,J=7.6Hz,1H),7.44(d,J=7.6Hz,2H),7.28-7.24(m,1H),7.14(d,J=7.6Hz,2H),1.36(s ,9H).
Synthetic example 3
[0034] (Z)-1-(4-methoxyphenylimine)naphtho[2,1-b]furan-2-one
[0035] Add 0.01mmol copper acetate, 0.3mmol acetic acid, 0.15mmol 2-naphthol, 0.1mmol N-(4-methoxyphenyl)glycine ethyl ester, solvent 1,2-dichloroethyl Alkanes 2.0mL. Heat to 120°C, keep stirring for 24h, stop the reaction, cool to room temperature, wash with saturated sodium chloride solution (25°C), extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography (washing Removal agent: petroleum ether / dichloromethane) to obtain the target product with a yield of 80%. 1 H NMR (400M, CDCl 3 ): δ8.99(d, J=8.4Hz, 1H), 8.03(d, J=8.8Hz, 1H), 7.88(d, J=8.4Hz, 1H), 7.68(t, J=7.6Hz, 1H ), 7.52(t, J=7.6Hz, 1H), 7.34-7.26(m, 3H), 6.97(d, J=8.8Hz, 2H), 3.09(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com