Triterpene-amino acid derivative as well as preparation method and application thereof
A kind of amino acid and derivative technology, applied in the field of triterpene natural amino acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] The synthesis of embodiment 1 oleanolic acid-valine conjugate
[0127]
[0128] Take 1 g of L-valine in a 50 ml reaction bottle, add 24 ml of methanol to dissolve it, then place the reaction bottle in an ice bath, slowly add 3 ml of thionyl chloride dropwise with a normal-pressure dropping funnel, and after the dropping Move to room temperature to react, and thin-layer detection after 3 hours, the ratio of developer: dichloromethane: methanol = 10:1, add a drop of ammonia water at the same time, and ninhydrin chromogenic reagent to develop color. Evaporate methanol and excess thionyl chloride directly to dryness, and the crude product can be directly used in the subsequent reaction without purification, but it is necessary to ensure that the product point on the thin layer is single. If the product point is not single, it needs to be purified by column chromatography.
[0129] Take 1g of oleanolic acid, dissolve it in 20ml of THF, add 844mg of TBTU and 339mg of DIEA ...
Embodiment 2
[0132] Example 2 Synthesis of oleanolic acid-histidine conjugates
[0133]
[0134] Take 1 g of L-histidine in a 50 ml reaction bottle, add 24 ml of methanol to dissolve it, then place the reaction bottle in an ice bath, slowly add 3 ml of thionyl chloride dropwise with a normal pressure dropping funnel, after the dropping Move to room temperature to react, after 3 hours TLC detection, developer ratio: dichloromethane: methanol = 10:1, add a drop of acetic acid at the same time, ninhydrin chromogenic reagent develops color. Evaporate methanol and excess thionyl chloride directly to dryness, and the crude product can be directly used in the subsequent reaction without purification, but it is necessary to ensure that the product point on the thin layer is single. If the product point is not single, it needs to be purified by column chromatography.
[0135] Take 539mg of L-histidine methyl ester hydrochloride, dissolve in THF, add 266mg of triethylamine, add 1g of oleanolic ac...
experiment example
[0140] The following are the experimental results of anti-influenza virus of some compounds of the present invention.
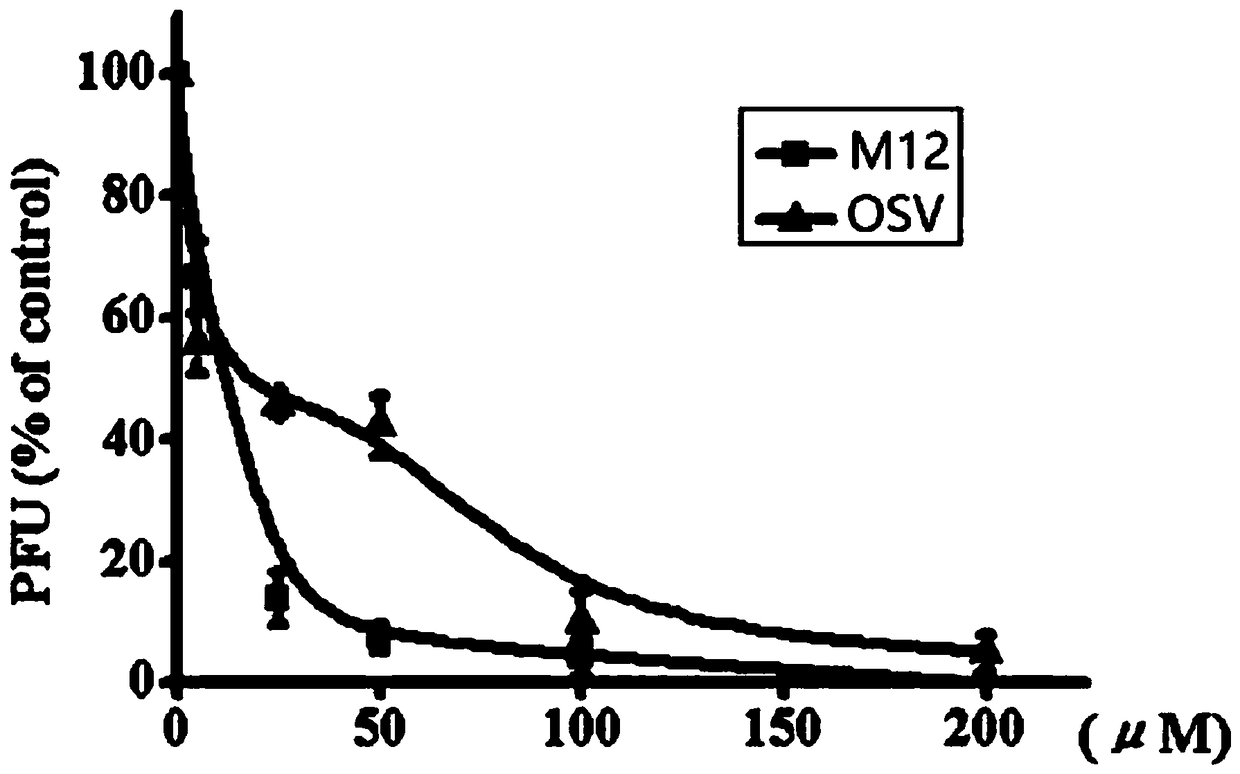
[0141] 1. M12 can effectively inhibit the replication of influenza virus. The CPE inhibition test and the plaque inhibition test proved that the compound M12 had a significant inhibitory effect on influenza virus, which was stronger than the positive drug ribavirin. The CPE inhibition assay showed that the EC of M12 against influenza virus 50 was 37.2µM, while the EC of the positive drug Tamiflu (oseltamivir phosphate, OSV-P) 50 EC of 41.9 µM, ribavirin (RBV) 50 is 50.3 µM (see Table 1). Plaque inhibition assays show the IC of M12 against influenza virus 50 figure 1 ). And the CC of M12 in A549, MDCK and 293T cells 50 All are greater than 100µM, indicating that the cytotoxicity of M12 is very small.
[0142] Table 1: Inhibitory activity of M12 against influenza virus (WSN) and its cytotoxicity analysis.
[0143]
[0144] a : CC 50 , the half cy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com