hg based on rhodamine derivatives 2+ Fluorescent probe and its preparation method and application
A fluorescent probe and derivative technology, applied in the field of fluorescence detection, can solve the problems of narrow pH range, poor selectivity, slow response speed, etc., and achieve the effects of high accuracy, good biocompatibility and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of Compound 2: Rhodamine B (4.79g, 10mmol) was added to a dry 100mL single-necked flask, and 30mL of ethanol was added to dissolve it, and 9mL of hydrazine hydrate (85%) was added dropwise under magnetic stirring, and heated to reflux for 12h Stop the reaction, cool the mixture solution to room temperature and distill off the solvent under reduced pressure, add 50mL of 1mol / L hydrochloric acid solution, then adjust its pH value to neutral with 1mol / L sodium hydroxide solution; add dichloromethane for extraction, Extracted three times, adding 30 mL each time, collected the dichloromethane phase, spin-dried the solvent, and dried to obtain 3.92 g of compound 2 as a khaki solid, with a yield of 86%.
[0058] Synthesis of Compound 3: Add Compound 2 (1.08 g, 2.4 mmol), Lowe’s Reagent (0.96 g, 2.4 mmol) and redistilled 40 mL of toluene to a 100 mL round-bottomed flask under nitrogen flow, and heat to reflux under magnetic stirring for reaction 4 Hour, after the mixt...
Embodiment 2
[0065] The measurement of embodiment 2 fluorescence intensity
[0066] A certain amount of compound 1 was dissolved in ethanol to obtain 1.0×10 -5 mol / L stock solution of compound 1.
[0067] Diluted step by step with 0.05mol / L Tris–HCl buffer solution of pH 7.24 1.0×10 -2 mol / L mercury nitrate solution to get 8×10 -7 -1.0×10 -3 mol / L Hg 2+ stock solution.
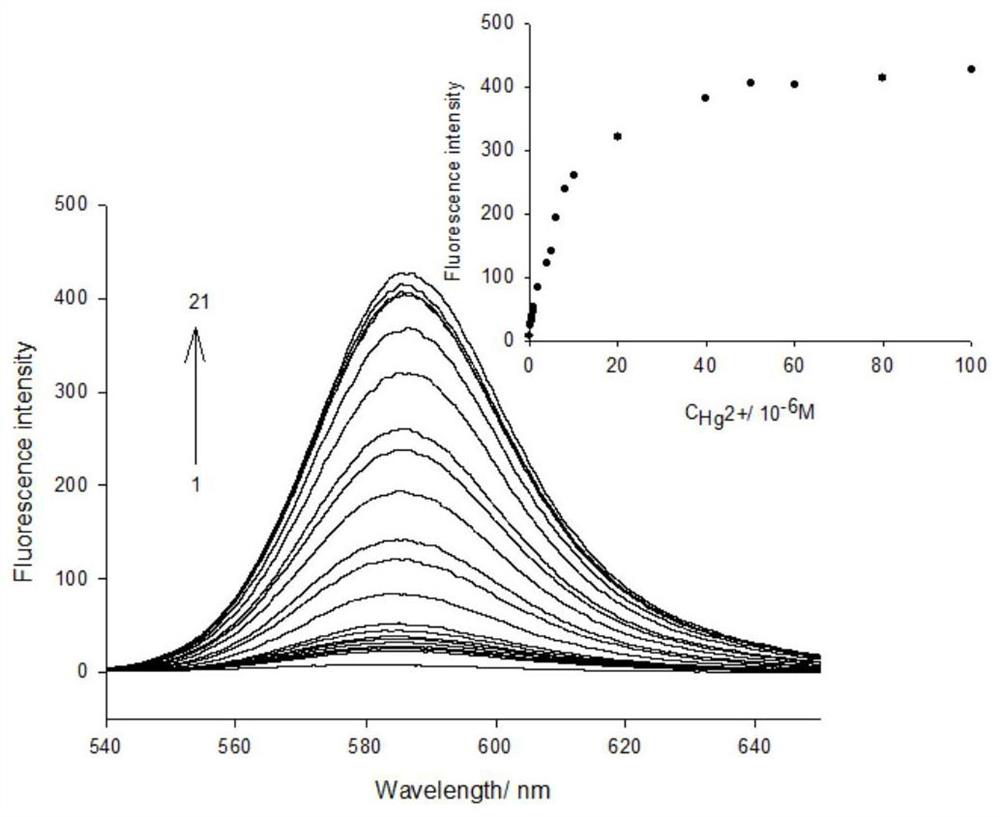
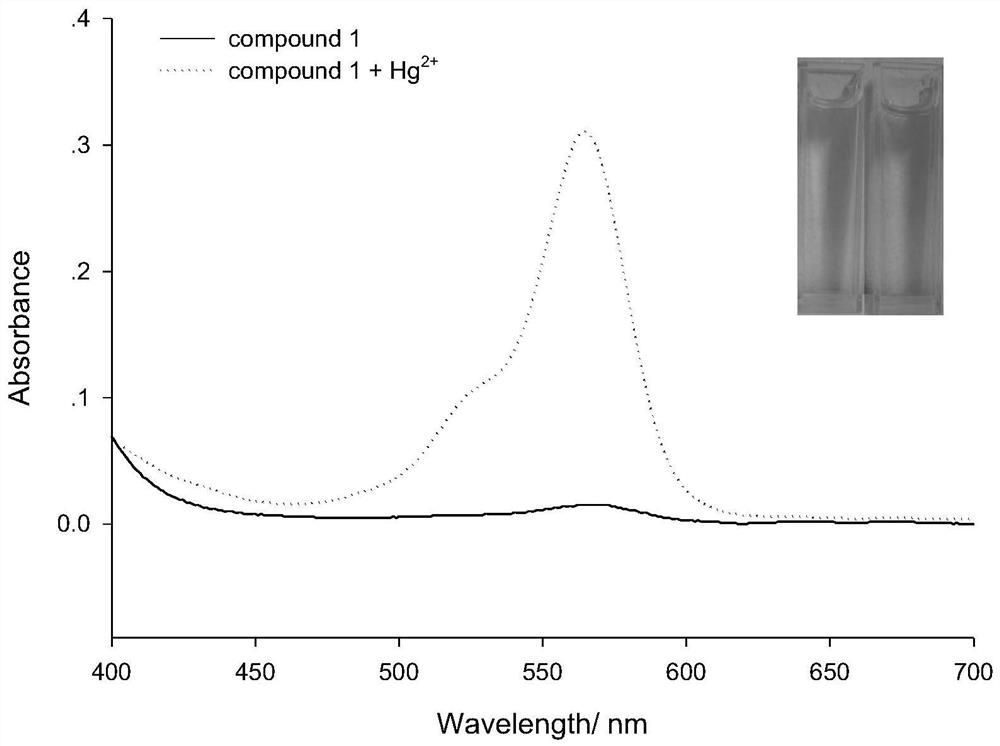
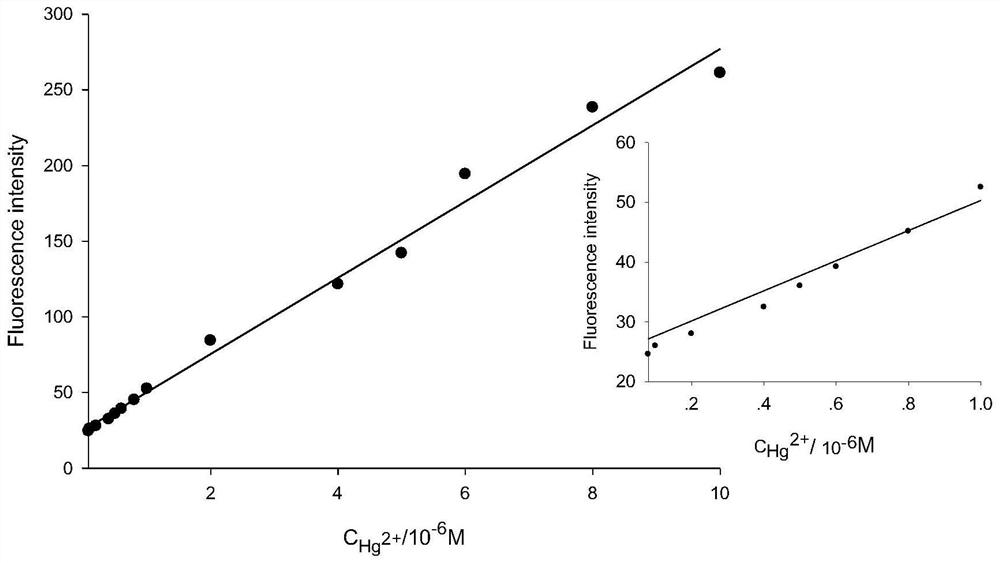
[0068] Precisely measure 12.50mL stock solution of compound 1 and 2.50mL of different concentrations of Hg 2+ Add the solution into a 25mL volumetric flask, and then dilute to 25mL with 0.05mol / L Tris-HCl solution. The solution thus obtained contained 5 x 10 -6 mol / L compound 1 and 8×10 -8 -1×10 -4 mol / L Hg 2+ solution, a blank solution of compound 1 was prepared under the same conditions, but without the addition of Hg 2+ . All solutions were stored at 4°C in the dark for future use.
[0069] When measuring the fluorescence intensity, the excitation wavelength is fixed at 520nm, the incident and exit slits ar...
Embodiment 3
[0081] Influence experiment of embodiment 3pH
[0082] The influence of different pH values (2.0-12.0) on the fluorescence intensity of compound 1 is detected, and the results are shown in Image 6 , Image 6 is a plot of the fluorescence emission intensity of compound 1 (5 μM) versus pH.
[0083] from Image 6 It can be seen that the fluorescence intensity of compound 1 remains basically unchanged when the pH is between 4.5 and 12.0, which indicates that the fluorescent probe is not affected by pH at pH 4.5-12.0 and can be used for the detection of actual samples. When the pH is less than 4.5, the fluorescence intensity value of compound 1 increases with the decrease of pH, which may be due to the ring-opening of the spirolactam structure of compound 1 under acidic conditions resulting in enhanced fluorescence.
[0084] Considering sensitivity, response time and practical application comprehensively, this application selects Tris-HCl buffer solution with pH 7.24 as the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com