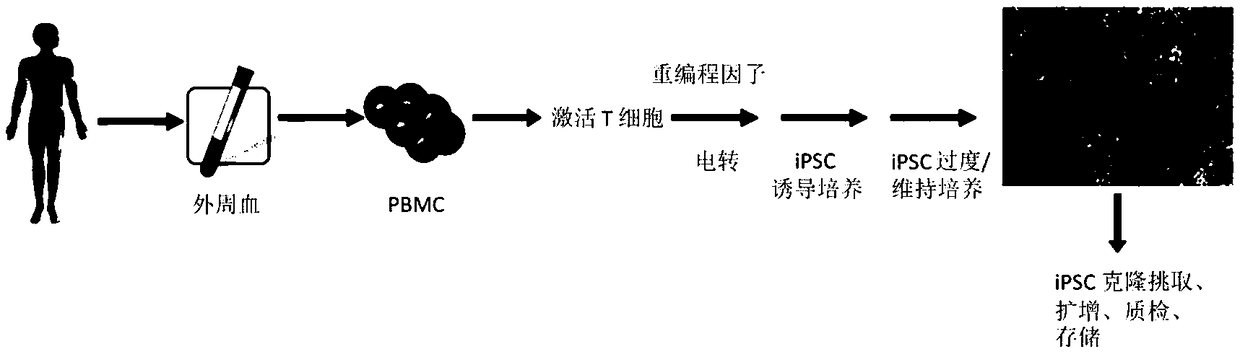
Reprogramming method for efficient inducing of T cells into multipotent stem cells
A technology for pluripotent stem cells and reprogramming, applied in animal cells, vertebrate cells, cell culture active agents, etc., can solve the problem of iPSC preparation technology failing to achieve large-scale preparation, and achieve the effect of high immune cell differentiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Design and Construction of Episomal Vector Expression System
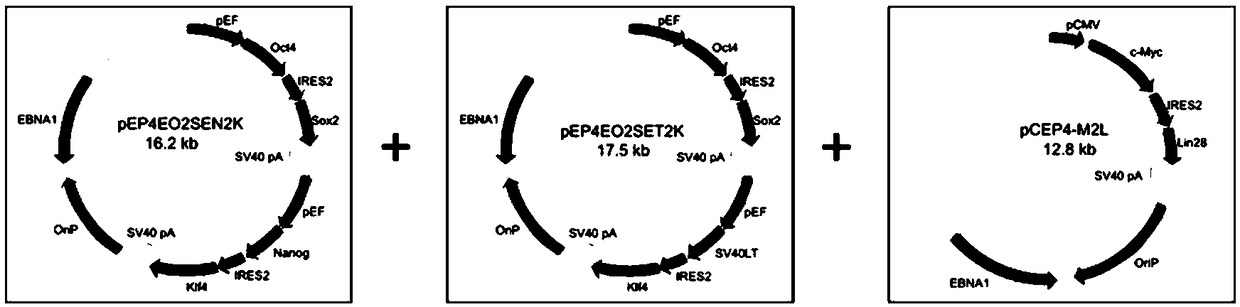
[0071] Such as figure 2 As shown, three kinds of episomal vectors were constructed in this example, all of which were amplified by direct polymerase chain reaction (PCR) from some or all of the open reading frames (ORFs) in the pluripotency determinant gene (using the The first and last 20-22 bases are used as primers), and the above ORF is inserted into the commercially available mammalian expression vector pCEP4 or related backbones containing OriP / EBNA1 to generate episomal vectors, all three episomal vectors contain at least An internal ribosome entry size (IRES), where the first vector is pEP4-E-O2S-E-N2K ( figure 2 A), which in turn comprises the first promoter, POU5F1, IRES2, SOX2, the second promoter, NANOG, IRES2 and KLF4; the second vector is pEP4-E-O2S-E-T2K ( figure 2 B), which sequentially comprises the third promoter, POU5F1, IRES2, SOX2, the fourth promoter, SV40LT, IRES2 and K...
Embodiment 2
[0072] Example 2: Primary isolation, culture and identification of T cell mononuclear cells
[0073] 1. Primary isolation and culture of T cell monocytes
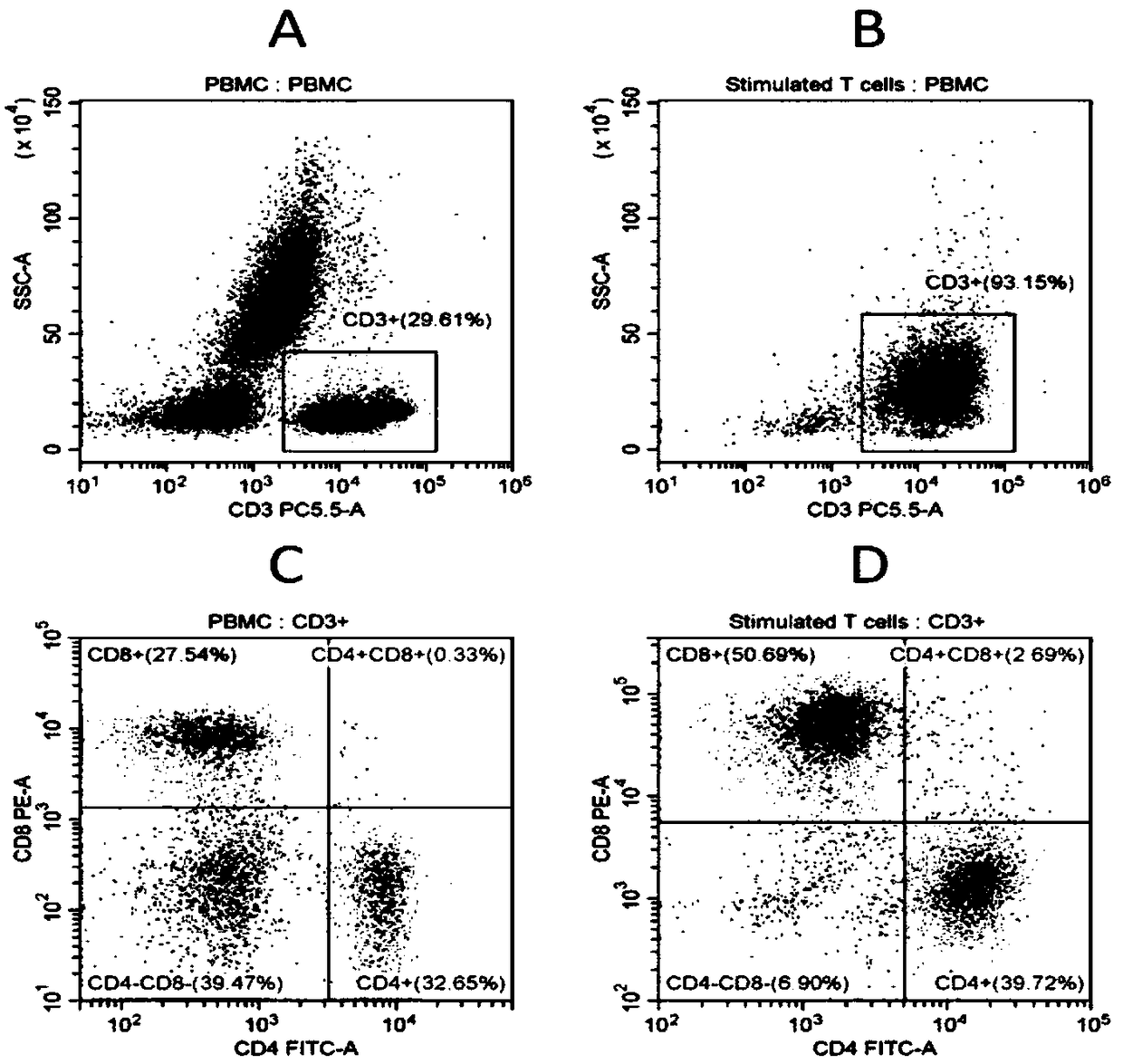
[0074] Collect 6ml of blood sample, transfer it to a lymphocyte separation tube, centrifuge, take the mononuclear cell layer, wash it twice with DPBS centrifugation, take a sample and count it, and use flow cytometry to detect the expression of the surface molecules CD3+ and CD4+CD8+, Detect its positive rate and average fluorescence intensity index respectively (experimental result such as image 3 Shown in A), take 6×10 according to the counting result 6 Cells were seeded in 3 wells of 6-well plate coated with activator, added T cell serum-free medium 2ml / well, placed at 37°C, 5% CO 2 Cultivation in an incubator constitutes the expansion culture system of the present invention, and each well is supplemented with 1-2 ml of fresh T cell serum-free medium on the first day of expansion.
[0075] The T cell serum-free mediu...
Embodiment 3
[0080] Example 3: Episomal vector-induced reprogramming
[0081] 1. Method
[0082] a. Take 0.5~4×10 T cells activated and cultured for 2 days in Example 2 6, using the pEP4-E-O2S-E-N2K, pEP4-E-O2S-E-T2K and pCEP4-LM-2L episomal vectors constructed in Example 1 to electrotransfect target cells, and then inoculated in hiPSC induction medium and Matrigel or vitronectin or other cell matrix-coated six-well plates, and the transfection contents of each plasmid DNA were pEP4-E-O2S-E-N2K: pEP4-E-O2S-E-T2K: pCEP4-LM -2L=1:1:1.
[0083] b. After 48 hours, half of the medium was replaced with fresh hiPSC induction medium, and the culture was continued until 10 days, and the medium was changed every other day, that is, reprogramming was performed on a feeder-free system.
[0084] The specific components of the pluripotent stem cell induction medium are:
[0085] On the basis of each liter of T cell serum-free expansion medium in Example 2, one or more of the following small molecule...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com