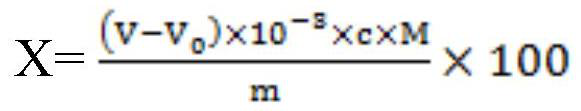A method for measuring iodine content in feed raw materials
A technology for feed raw materials and iodine content, which is applied in the direction of chemical analysis by titration method, can solve the problems of unsuitable determination of calcium iodate iodine content, high operation difficulty, complicated steps, etc. Convenience and the effect of improving detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1 Preparation of reagents required for the test
[0035] 1.1 Sulfuric acid solution: 20% H 2 SO 4 solution;
[0036] 1.2 Ferrous sulfate solution: 50g / L FeSO 4 solution;
[0037] 1.3 Standard solution of sodium thiosulfate: c(Na 2 S 2 o 3 )≈0.1mol / L;
[0038] 1.4 Starch indicator solution: 10g / L.
[0039] 2 Preparation of samples
[0040] Take about 50g of the calcium iodate sample to be tested, put it in an airtight container, and label it.
[0041] 3 Determination of samples
[0042] Weigh 0.5-3g of calcium iodate sample (accurate to 0.0002g, wherein, 1% calcium iodate weighs about 3g, 2% calcium iodate weighs about 2g, 5% calcium iodate weighs about 1g, 10% Weigh about 0.5g of calcium iodate), put it in a 250mL beaker, add 20mL of 20% sulfuric acid solution, 20mL of water, heat and boil on an electric furnace to dissolve the sample, filter it with a medium-speed quantitative filter paper while it is hot, and dehydrate it with 30mL of hot water. Wash the f...
Embodiment 2
[0073] 1) Instruments and equipment
[0074] Analytical balance (one set accurate to 0.0001g), electric furnace (2500W), 250mL beaker, 250mL iodine measuring bottle, funnel, medium-speed quantitative filter paper, 25mL burette single channel.
[0075] 2) Environmental requirements
[0076] Room temperature: 20-25°C.
[0077] In this embodiment, the detection process of the experimental group is the same as that in Embodiment 1.
[0078] Get the 1% calcium iodate sample and divide it into multiple parts, with the improved chemical industry standard "HG / T 2418-2011 Feed Grade Calcium Iodate" as the control group 1, with the national standard "GB / T 13882-2010 Feed Iodine Determination of Ferric Thiocyanate-Nitrite Catalytic Kinetic Method" as the control group 2, with this method as the experimental group, respectively compare three methods to the determination results of the iodine content in the same batch of 1% calcium iodate samples. Wherein, in the control group 1 method,...
Embodiment 3
[0088] A method for measuring iodine content in feed materials, the method may further comprise the steps:
[0089] 1) the calcium iodate sample, H 2 SO 4 The mass percentage content is 22% sulfuric acid solution, water is heated after mixing, and is taken off after being heated to boiling. Filtrate while hot afterwards, wash, collect filtrate and washing liquid and mix, obtain test solution, wherein, the volume ratio of sulfuric acid solution and water is 1:0.9, adds 3g calcium iodate sample in every 20mL sulfuric acid solution;
[0090] 2) Add the ferrous sulfate solution whose mass volume concentration is 45g / L, and the starch indicator solution whose mass volume concentration is 12g / L into the test solution, then titrate with sodium thiosulfate standard solution until the test solution becomes Colorless, the iodine content is calculated, wherein the volume ratio of the ferrous sulfate solution to the sulfuric acid solution in step 1) is 1:10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com