(FeaCobNic)xMyRz oxygen evolution catalytic material and application to anode oxygen evolution electrode
A catalytic material and oxygen evolution electrode technology, applied in electrodes, electrolysis components, electrolysis process, etc., can solve the problems of electrode reaction deviation from equilibrium electrode potential, catalytic activity needs to be improved, limit the development of electrolytic water industry, etc., to reduce oxygen evolution Effects of overpotential, excellent catalytic activity, and improved energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
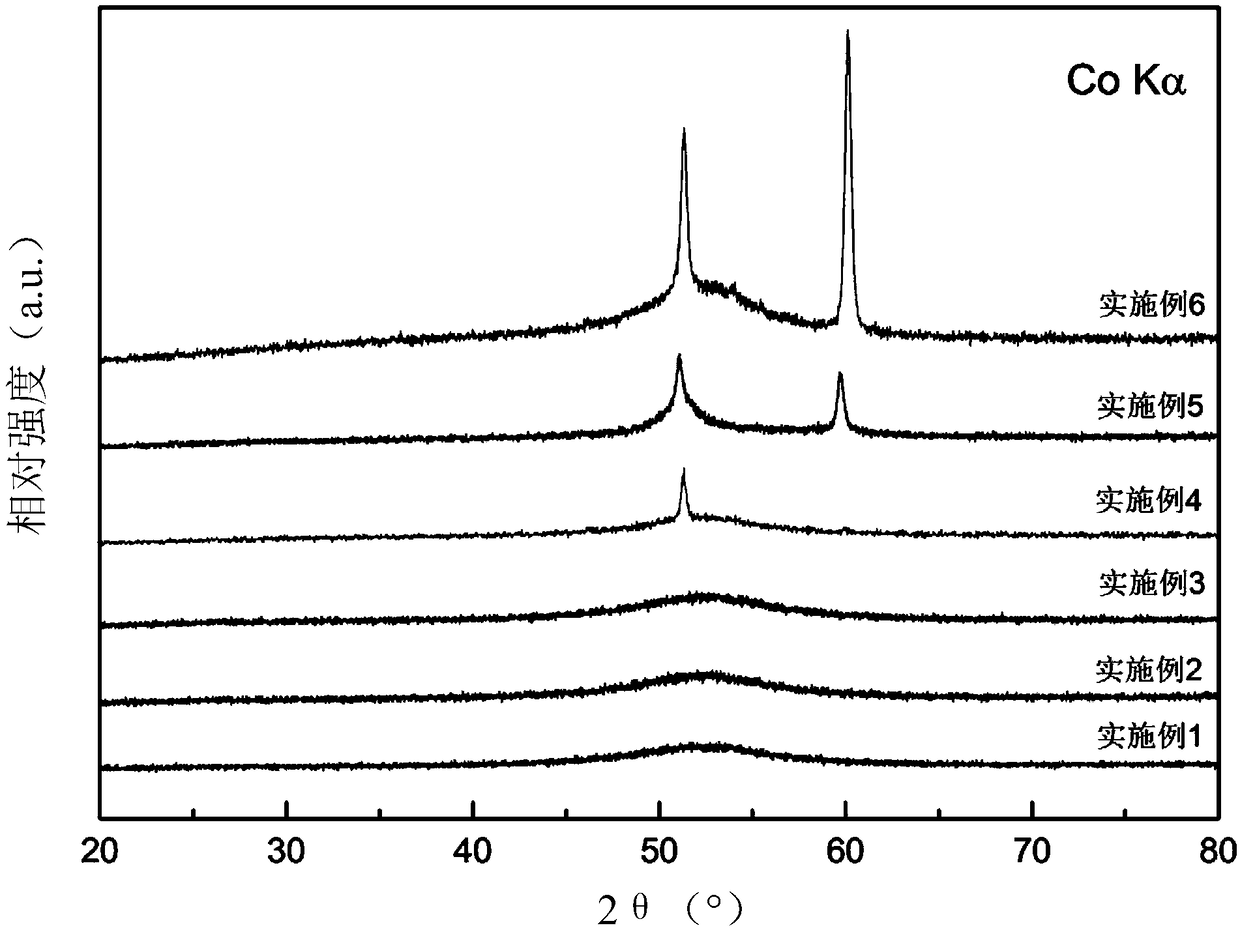
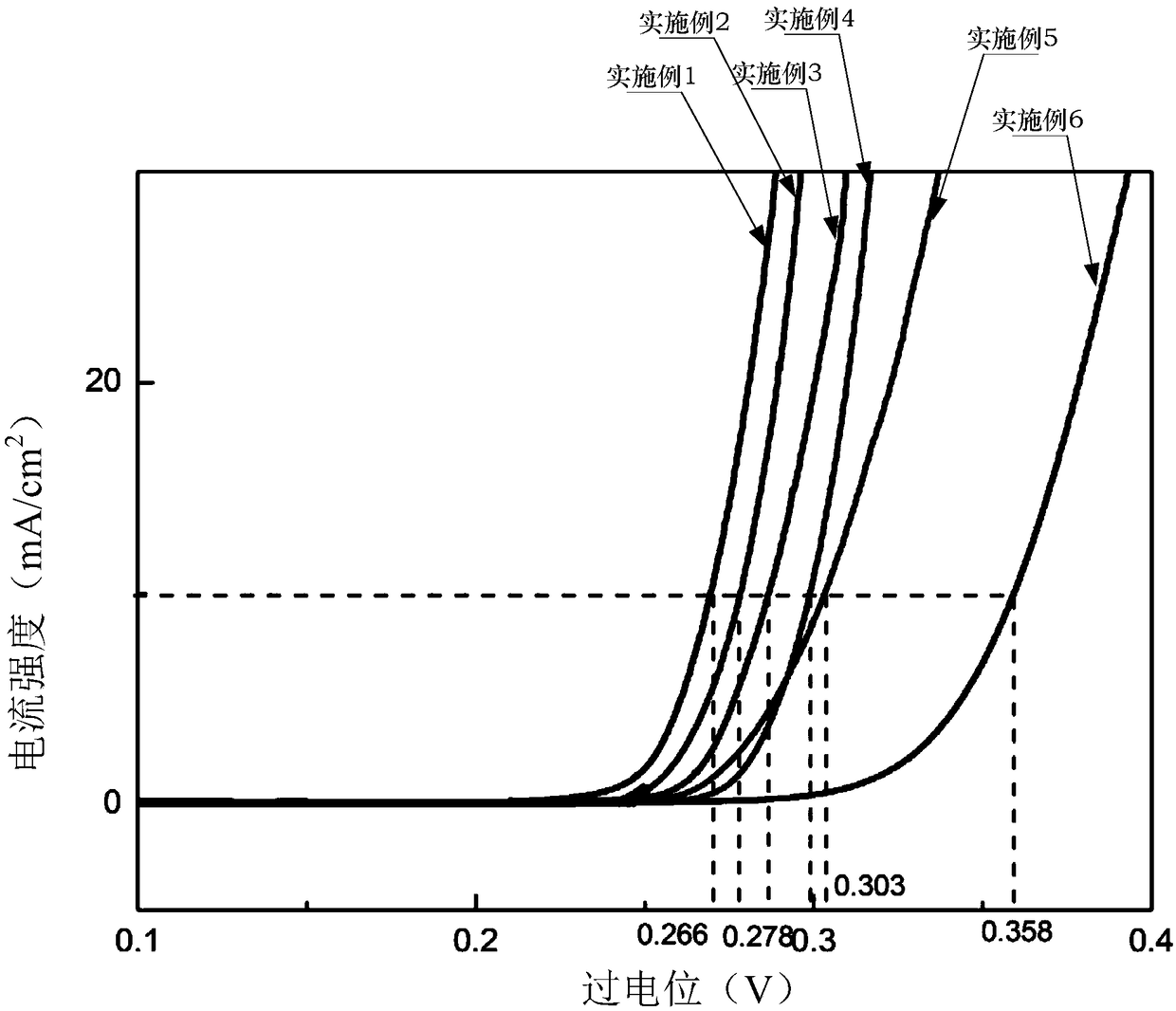
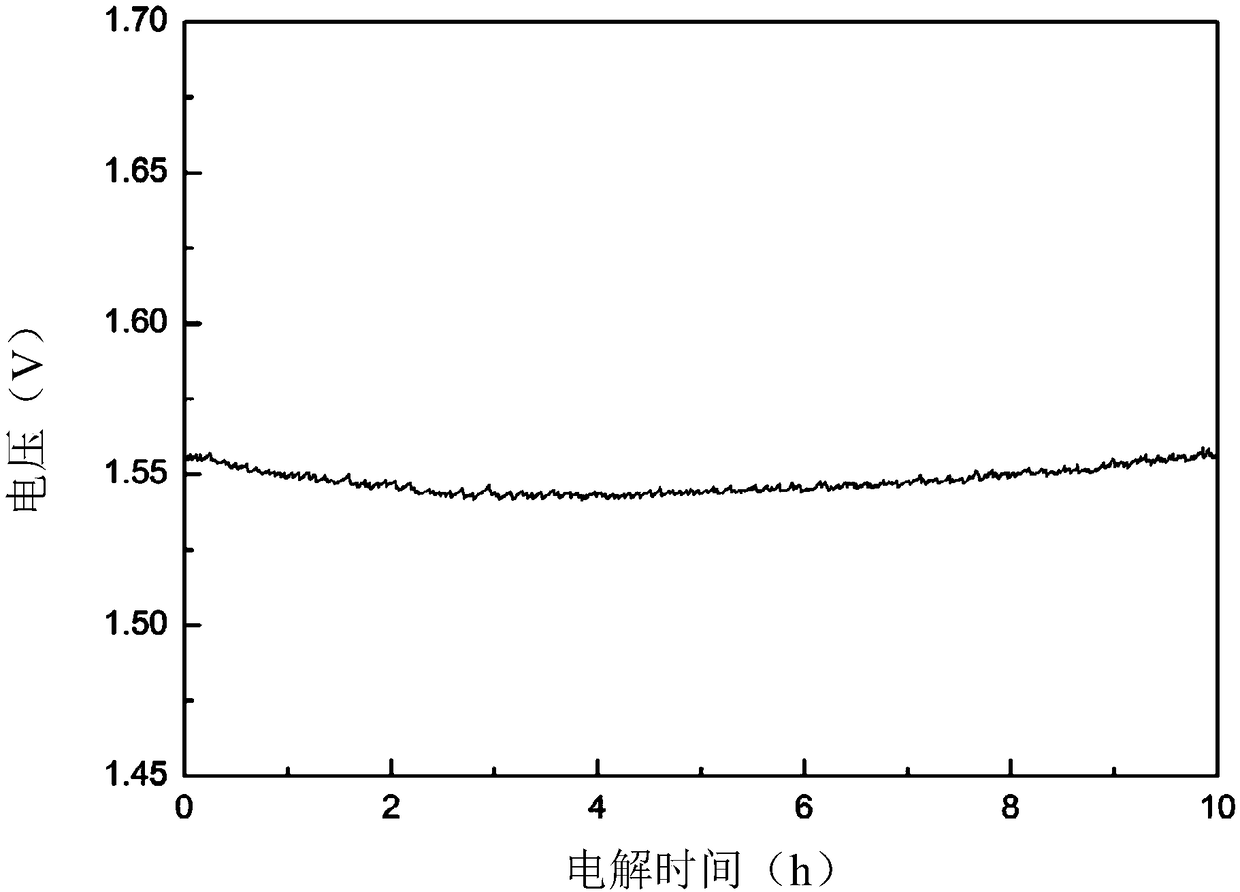
Examples
Embodiment 1
[0073] Preparation of Fe 40 co 8 Ni 40 B 10 Ru 2 Oxygen Evolution Catalytic Amorphous Alloy Electrode
[0074] Step 1: Dispensing ingredients according to the target ingredients;
[0075] According to the atomic percentage of Fe 40 co 8 Ni 40 B 10 Ru 2 The target composition is to weigh the elemental elements of Fe, Co, Ni, B, Ru, among which the B element can be replaced by FeB pre-alloyed, and the mass percentage purity of each element is not less than 99.0%;
[0076] Step 2: Melting the master alloy;
[0077] Mix the elements of Fe, Co, Ni, FeB, and Ru weighed in step 1 evenly, and melt them in a vacuum arc melting furnace to obtain Fe 40 co 8 Ni 40 B 10 Ru 2 target alloy ingot;
[0078] Melting parameters: the melting protective atmosphere is argon with a mass percentage of 99.999%;
[0079] Vacuum degree is 8.5×10 -3 Pa;
[0080] The melting current is 150A;
[0081] Melting time: 2 minutes for each smelting, 4 times for smelting;
[0082] Step 3: Pre...
Embodiment 2
[0098] Preparation of Fe 35 co 12 Ni 35 Si 14 Nb 4 Oxygen Evolution Catalytic Amorphous Alloy Electrode
[0099] Step 1: Ingredients
[0100] According to Fe 35 co 12 Ni 35 Si 14 Nb 4 The target composition weighs the elemental elements of Fe, Co, Ni, Si, and Nb, and the mass percentage purity of each element is not less than 99.0%;
[0101] Step 2: Melting the master alloy
[0102] Mix the Fe, Co, Ni, Si, and Nb elements weighed in step 1 evenly, and melt them in a vacuum arc melting furnace to obtain Fe 35 co 12 Ni 35 Si 14 Nb 4 target alloy ingot;
[0103] Melting parameters: the melting protective atmosphere is argon with a mass percentage of 99.999%;
[0104] Vacuum degree is 9×10 -3 Pa;
[0105] The melting current is 120A;
[0106] Melting time: 2 minutes for each smelting, 4 times for smelting;
[0107] Step 3: Preparation of target alloy strips by melt spin quenching method
[0108] The master alloy obtained in step 2 is mechanically broken into ...
Embodiment 3
[0123] Preparation of Fe 32 co 14 Ni 32 Si 8 P 8 Nb 4 Mo 2 Oxygen Evolution Catalytic Amorphous Alloy Electrode
[0124] Step 1: Ingredients
[0125] According to Fe 32 co 14 Ni 32 Si 8 P 8 Nb 4 Mo 2 The target composition weighs the elemental elements of Fe, Co, Ni, Si, P, Nb, and Mo (where P can be replaced by FeP), and the mass percentage purity of each element is not less than 99.0%;
[0126] Step 2: Melting the master alloy
[0127] Mix the Fe, Co, Ni, Si, P (or FeP), Nb, and Mo elements weighed in step 1 evenly, and melt them in a vacuum arc melting furnace to obtain Fe 32 co 14 Ni 32 Si 8 P 8 Nb 4 Mo 2 target alloy ingot;
[0128] Melting parameters: the melting protective atmosphere is argon with a mass percentage of 99.999%;
[0129] Vacuum degree is 9×10 -3 Pa;
[0130] The melting current is 120A;
[0131] Melting time: 2.5 minutes for each smelting, 4 times for smelting;
[0132] Step 3: Preparation of target alloy strips by melt spin que...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com