Pharmaceutical formulation and method of preparing the same
A preparation and medicine technology, applied in the direction of medicine formulation, drug combination, drug delivery, etc., can solve the problems of fast synthesis time, limited reagent feasibility, etc., achieve the effect of efficient preparation and avoid column purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
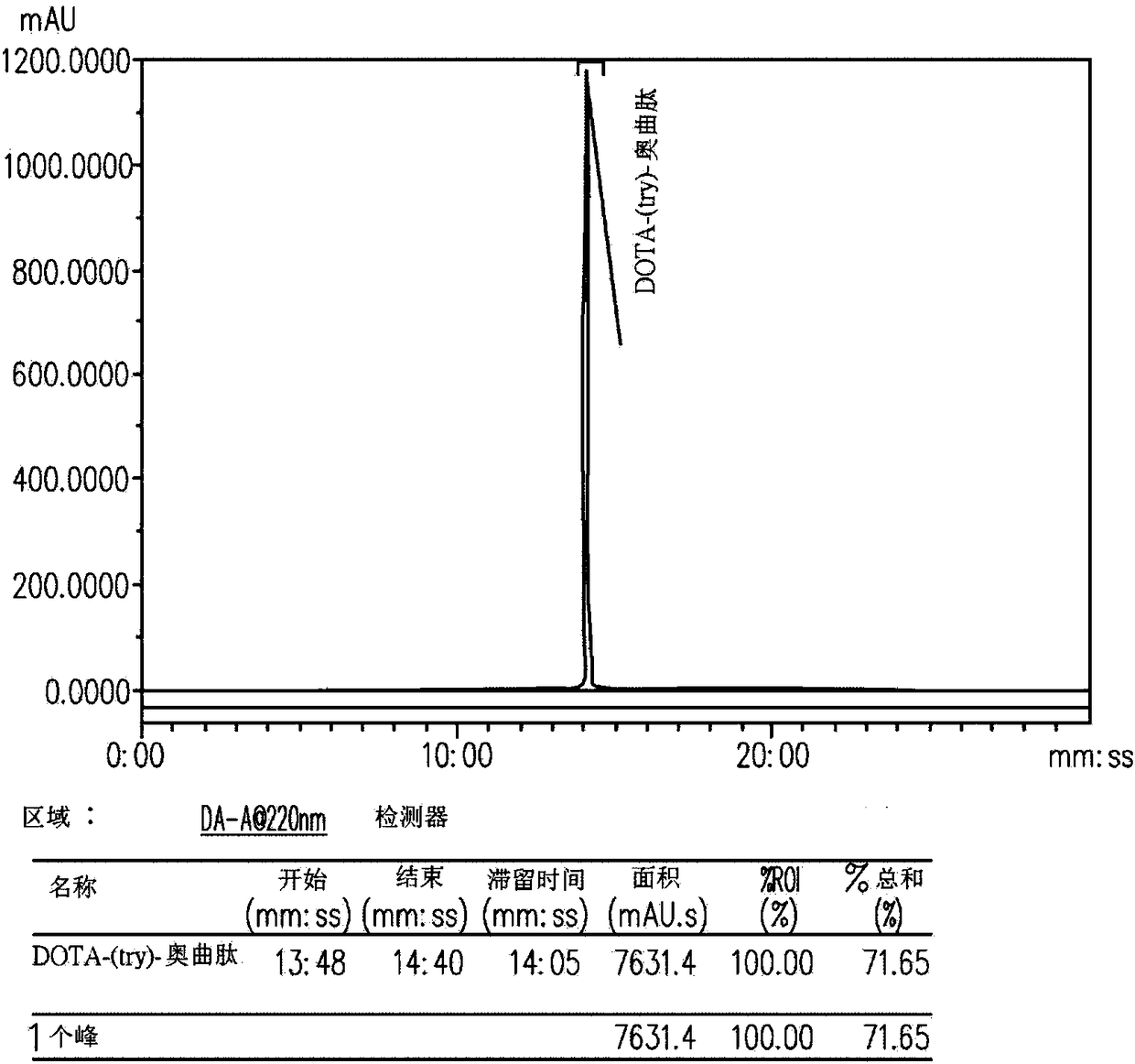
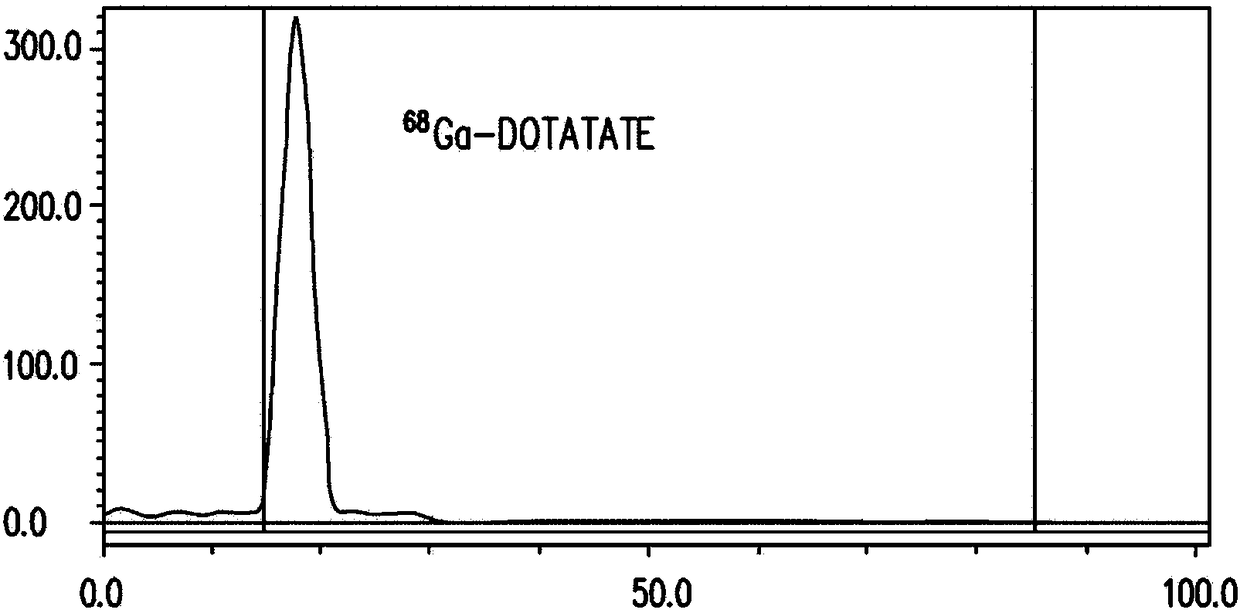
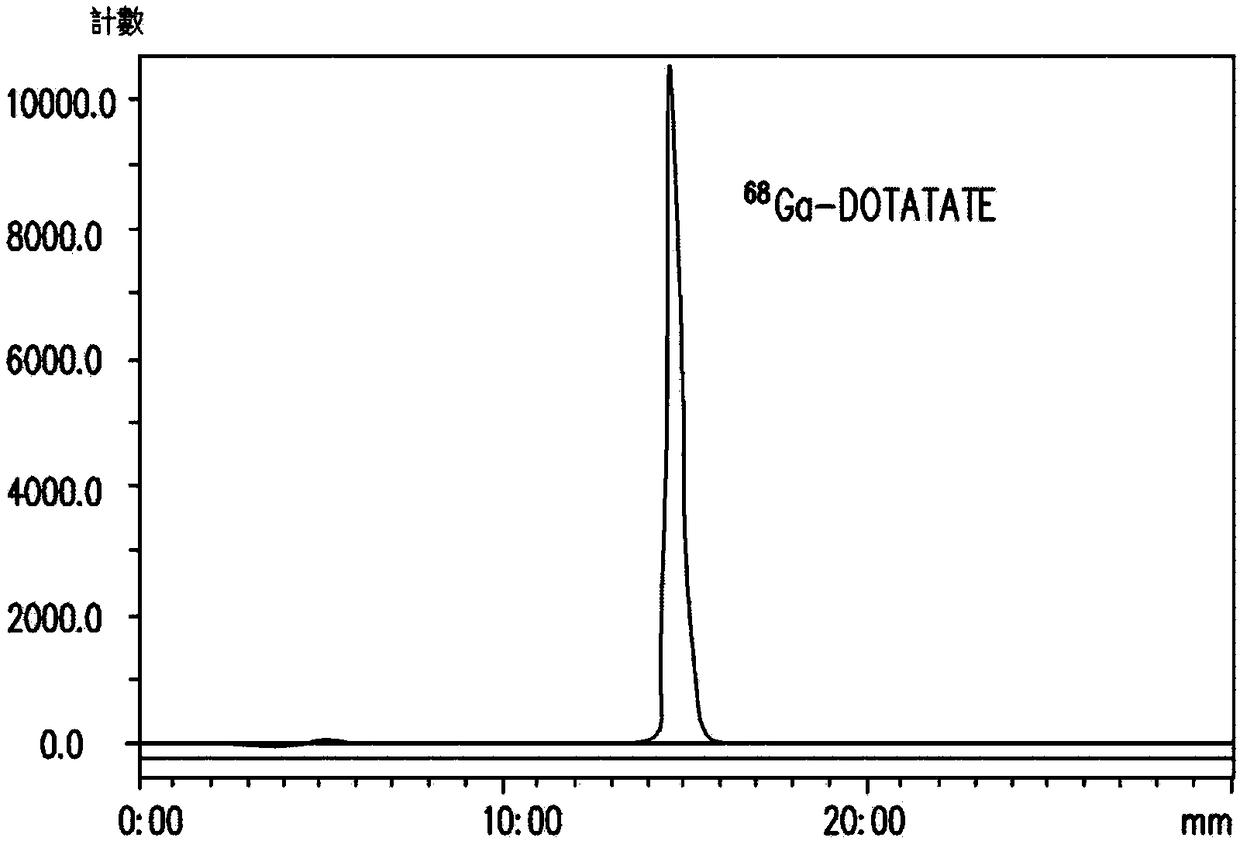
[0061] Example 1: 68 Synthesis of Ga-DOTATATE
[0062] measured from 68 Ge / 68 Ga generator's 68 Ga-Elution File
[0063] In this example, HCl (ranging from 0.01N-1N) was used to elute from 68 Ge / 68 Ga generator obtained 68 GaCl 3 . For example, eluting with 0.3N and 0.6N HCl (10mL) from 68 Ge / 68 Ga generator's 68 GaCl 3 . Then on the next day, the elution volume (0.3N or 0.6N HCl, 6 mL) was divided into 12 tubes (0.5 mL / tube). The radioactivity of each tube was counted and the results are shown in Table 1 below.
[0064] Table 1: 68 Ga Elution Activity Profile
[0065]
Eluent 1 (0.6N HCl(aq))
Eluent 2 (0.3N HCl(aq))
1st aliquot
0.41uCi
0.79mCi
2nd fraction
0.35uCi
0.95mCi
3rd aliquot
0.903mCi
7.22mCi
4th aliquot
7.87mCi
3.10mCi
5th aliquot
7.55mCi
2.45mCi
6th aliquot
6.25mCi
1.51mCi
7th fraction
0.851mCi
0.73mCi
8th fraction
0....
example 2
[0083] Example 2: Positron Emission Tomography (PET) Imaging Study
[0084] to demonstrate the mix 68 Formulations of Ga-DOTATATE and the transchelator β-cyclodextrin (CD) were more effective than either alone 68 Ga-DOTATATE has the same or better imaging quality, and two animal models (colorectum and pancreas) with known neuroendocrine tumors were selected for imaging. Specifically, make 68 Ga-N4-tyrosine was incubated with plasma for up to 3 hours. Combine image data with 18 F-FDG (gold standard) and 68 Ga-DOTA (negative control) was used for comparison.
[0085] Briefly, imaging studies were performed using athymic nude mice (15±2 g) bearing human tumors (in the hind legs) derived from colorectal and pancreatic cell lines. Studies were performed when tumors were approximately 0.5 cm in diameter 21 to 28 days after inoculation. Nuclide scintigraphic images were obtained from micro-PET (Inveon) embedded in the gantries coordinate PET / CT data acquisition. each animal i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com