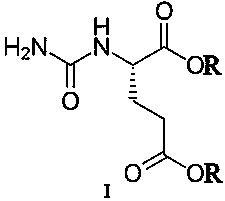
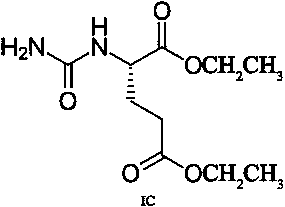
Transdermal absorption preparation for treating diabetic peripheral neuropathic pain
A technology for transdermal absorption preparations and diabetes, which is applied in the direction of nervous system diseases, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of high price, high curative effect, and low side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Antidiabetic Peripheral Neuralgia Gel and Its Preparation
[0030] Gel formulation: Pregabalin 70g, compound (IA) 10g, carbomer 940 21g, glycerin 78g, Tween 80 21g triethanolamine 32g, laurocaprolactone 7g and distilled water 1261g.
[0031] Preparation method: Take the prescribed amount of Carbomer 940 and add 1029g of distilled water, let stand overnight to fully swell; add the prescribed amount of glycerin to grind to moisten, and add the prescribed amount of triethanolamine to grind into a transparent gel matrix. Take the remaining amount of distilled water, prescription Tween 80, laurocaprazine, compound (IA), and pregabalin, stir, add to the gel base and stir at 90 rpm for 8 minutes, and divide into medicinal ointment tubes to obtain.
Embodiment 2
[0032] Example 2 Antidiabetic Peripheral Neuralgia Gel and Its Preparation
[0033] Gel formulation: gabapentin 90g, compound (IA) 10g, carbomer 940 21g, glycerin 78g, Tween 80 21g triethanolamine 32g, laurocaprolactone 7g and distilled water 1241g.
[0034] Preparation method: Take the prescribed amount of Carbomer 940 and add 1029g of distilled water, let stand overnight to fully swell; add the prescribed amount of glycerin to grind to moisten, and add the prescribed amount of triethanolamine to grind into a transparent gel matrix. Take the remaining amount of distilled water, prescription Tween 80, laurocaprazine, compound (IA), and gabapentin and stir, add to the gel base and stir at 60 rpm for 12 minutes, then put them into medicinal ointment tubes.
Embodiment 3
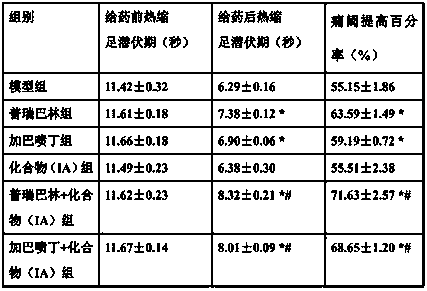
[0035] Example 3 Investigation of Drug Effects of Antidiabetic Peripheral Neuralgia Prescription
[0036]Healthy male SD rats, weighing 190-200 g, were raised in the SPF laboratory. Streptozotocin was dissolved in sodium citrate buffer solution and prepared immediately. After the rats were fasted for 24 hours, each rat was intraperitoneally injected with 12 mg of streptozotocin. On the 14th day after streptozotocin injection, the diabetic rats were taken to measure the reaction time of licking, screaming and jumping in the rats by the hot plate method, that is, the heat shrinkage latency period before administration, and the temperature of the hot plate was 51- 52°C. Each rat was repeatedly measured 3 times, with an interval of 15 minutes between the two measurements, and the mean value of the 3 measurement results represented the thermal paw withdrawal latency of the rat before administration.
[0037] On the 15th day after streptozotocin injection, the rats were randomly ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com