Non-noble metal-based water-electrolysis oxygen evolution reaction electrocatalyst and preparation method thereof
An electrocatalyst and non-precious metal technology, which is applied in the field of non-precious metal-based electrocatalysts for water electrolysis and oxygen evolution reaction, can solve the problems of unstable activity of ultrafine nanoclusters, difficult chemical preparation process, loss of size advantages, etc. Excellent long-term stability, excellent stability, simple and easy preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Preparation of FeOOH / LDH: 0.5 mmol nickel chloride hexahydrate, 10 mmol urea and 5 mmol were dissolved in 60 milliliters of deionized water; 100 milligrams of 316L stainless steel nanopowder was washed with water and ethanol and dried. Get 100 mg and add it to the above solution, ultrasonically disperse for 10 minutes (ultrasonic instrument is an ultrasonic cleaner KQ-100TDE) and mechanically stir for 10 minutes with a rotating speed of 100 rpm; the resulting suspension is transferred to 80 ml of Teflon Lining, placed in a stainless steel autoclave, heated at 120°C for 15 hours, and then cooled to room temperature; the obtained product was separated with a magnet and washed with deionized water, and the product that could not be adsorbed by the magnet was loaded highly dispersed iron oxyhydroxide Nanoclusters, repeated three times, were subsequently dried in a 60 °C drying oven.
Embodiment 2
[0039]Dissolve 0.5 mmol nickel chloride hexahydrate, 10 mmol urea and 5 mmol in 60 ml of deionized water; wash and dry the 316L stainless steel nanopowder with water and ethanol, add 10 mg to the above solution, and ultrasonically disperse 10 minutes (ultrasonic instrument is ultrasonic cleaning instrument KQ-100TDE) and with rotating speed is 100 revs per minute mechanical stirring 10 minutes; The suspension obtained is transferred in 80 milliliters of Teflon liners, puts into stainless steel autoclave, in Heated at 120°C for 15 hours, and then cooled to room temperature; the obtained product was separated by a magnet and washed with deionized water, and the product that could not be adsorbed by the magnet was a loaded highly dispersed iron oxyhydroxide nanocluster, repeated three times, and then heated at 60°C Dry in a drying oven.
Embodiment 3
[0041] Dissolve 0.5 mmol nickel chloride hexahydrate, 10 mmol urea and 5 mmol in 60 ml deionized water; wash and dry 316L stainless steel nanopowder with water and ethanol, add 300 mg to the above solution, and ultrasonically disperse 10 minutes (ultrasonic instrument is ultrasonic cleaning instrument KQ-100TDE) and with rotating speed is 100 revs per minute mechanical stirring 10 minutes; The suspension obtained is transferred in 80 milliliters of Teflon liners, puts into stainless steel autoclave, in Heated at 120°C for 15 hours, and then cooled to room temperature; the obtained product was separated by a magnet and washed with deionized water, and the product that could not be adsorbed by the magnet was a loaded highly dispersed iron oxyhydroxide nanocluster, repeated three times, and then heated at 60°C Dry in a drying oven.
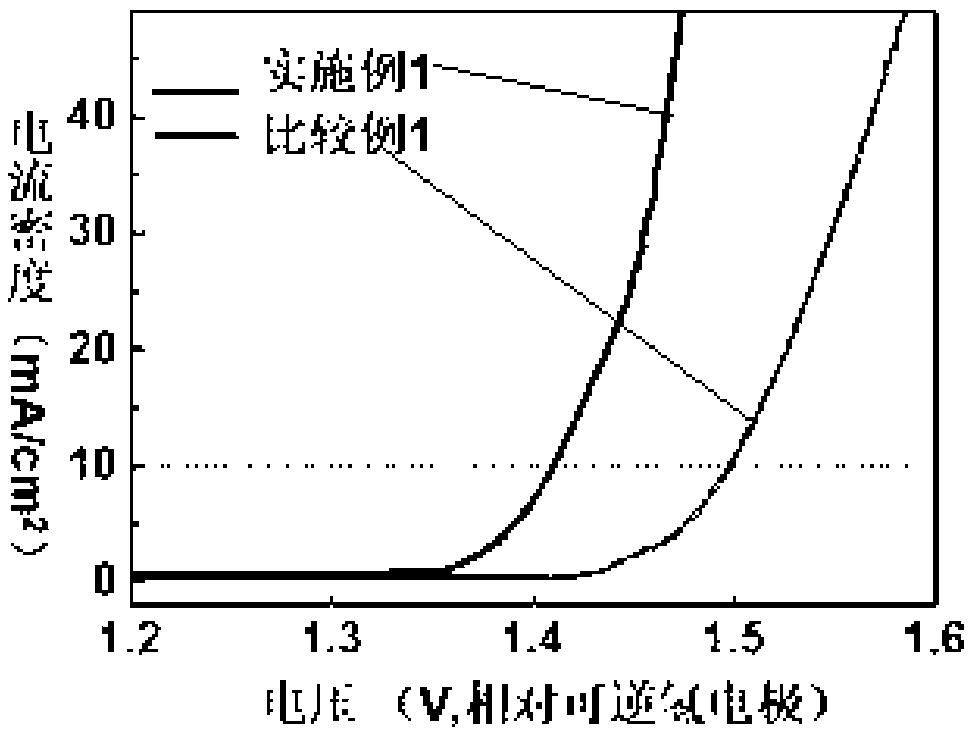
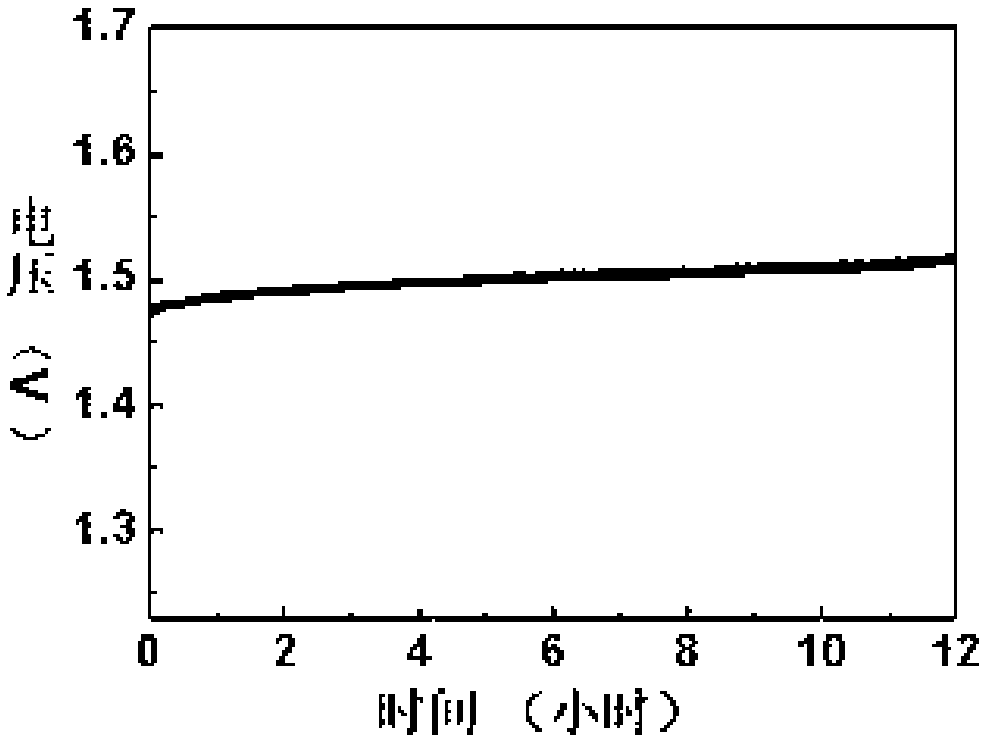
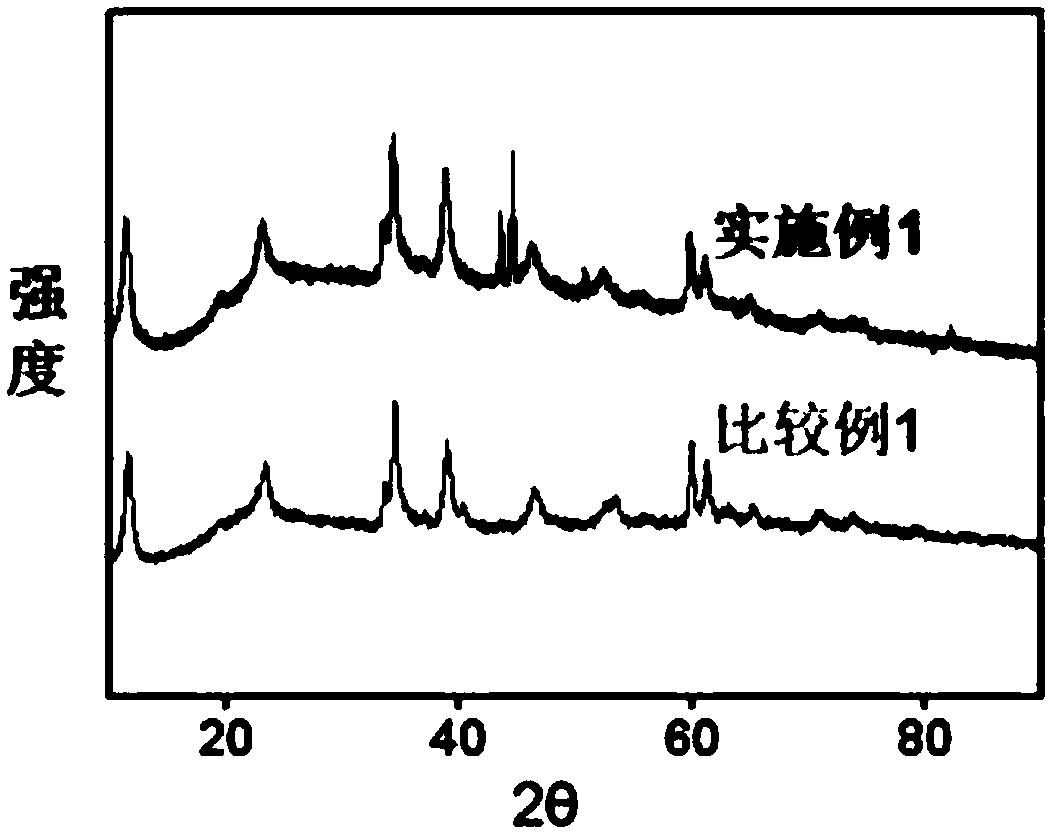
[0042] Embodiment 4 The electrochemical performance test of the electrocatalyst prepared by embodiment 1-3
[0043] Catalyst ink was prepared by di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com