Branched paraffin degradation biomarker and synthetic method thereof
A biomarker, branched-chain alkane technology, applied in the field of organic compound synthesis, can solve the problems of large differences, no standard substance control, lack of mass spectrometry information, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
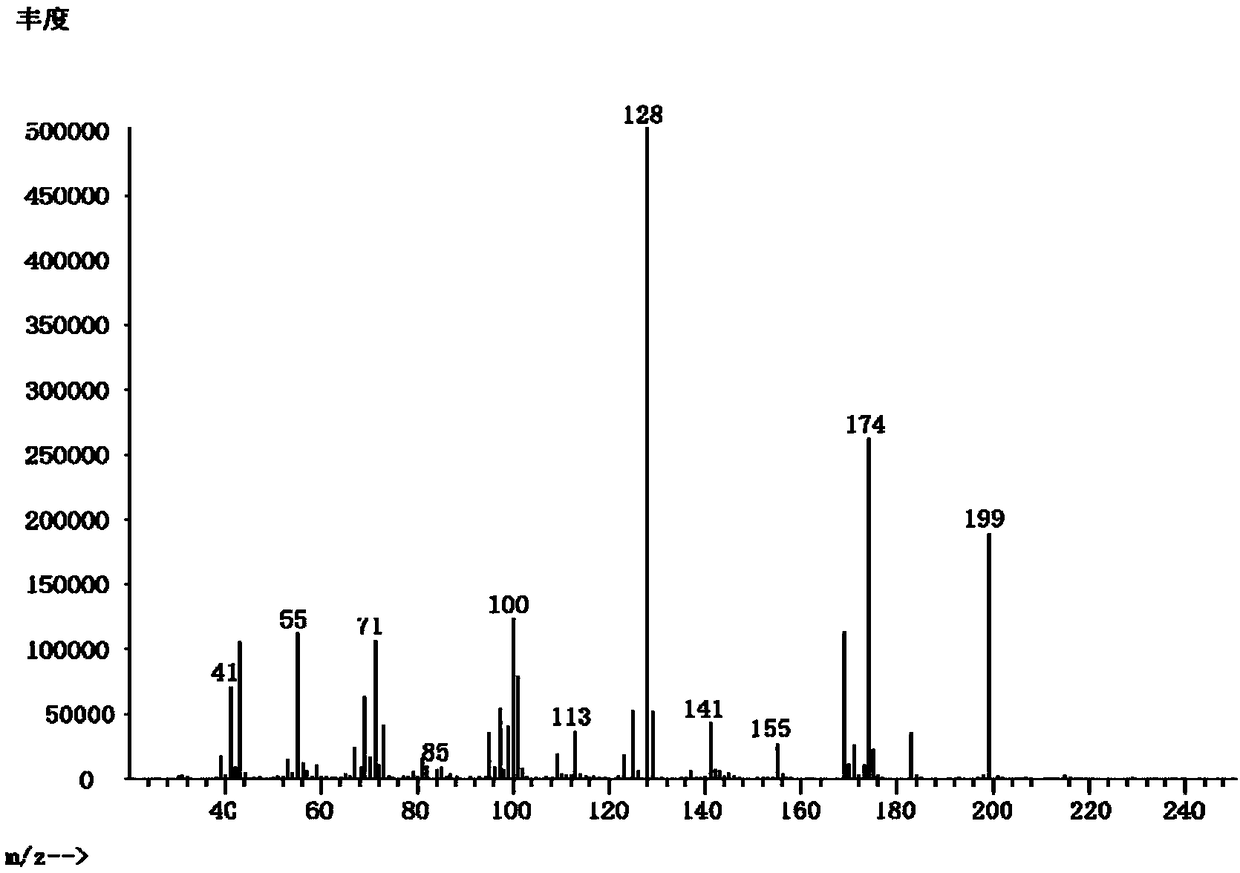
[0033] Example 1: 2-(1,1-Dimethylpropyl)succinic acid
[0034] (1) Add 0.42 mol of acetone (re-distilled or add anhydrous magnesium sulfate to remove water) dropwise to 1-propylmagnesium bromide in tetrahydrofuran under ice-bath conditions, and stir overnight at room temperature. Under the condition of ice bath, 0.48 mol of deionized water was added dropwise to quench the reaction. Filter, extract with n-hexane (10 mL×3), combine the organic phases, dry and remove the solvent by rotary evaporation to obtain 2-methyl-2-butanol.
[0035] (2) Dissolve 0.4 mol of anhydrous zinc chloride in 0.4 mol of concentrated hydrochloric acid, and cool for later use. Add 0.4mol 2-methyl-2-butanol, shake slightly, and take the organic phase to obtain 2-methyl-2-chlorobutane.
[0036] (3) Add 30 mL of anhydrous tetrahydrofuran into a 100 mL three-necked flask, add 0.44 mol of sodium hydride in an ice bath, stir well, then add 0.4 mol of 1,1,2-ethanetricarboxylic acid triethyl ester, and conti...
Embodiment 2
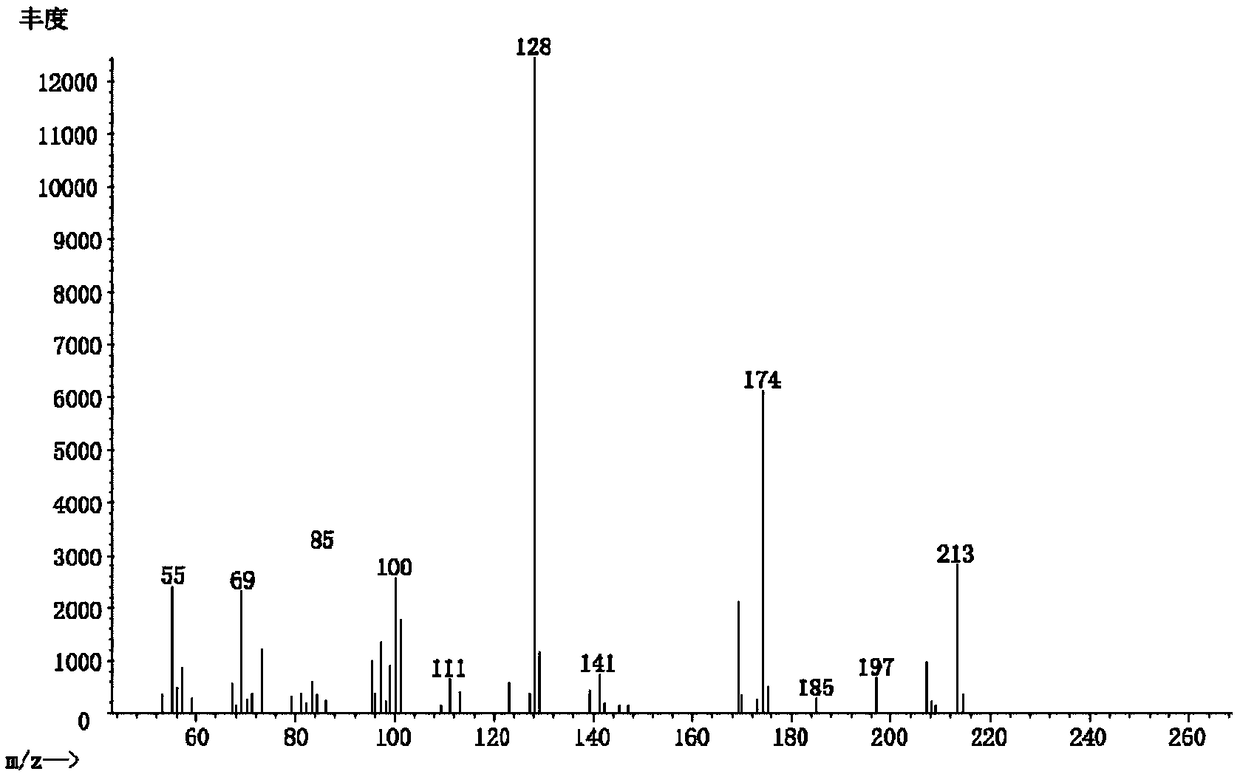
[0039] Example 2: 2-(1,1-dimethylbutyl)succinic acid
[0040](1) Add 0.44 mol of acetone (redistilled or add anhydrous magnesium sulfate to remove water) dropwise to 1-butylmagnesium bromide in tetrahydrofuran solution under ice bath condition, and stir overnight at room temperature. Under the condition of ice bath, 0.48 mol of deionized water was added dropwise to quench the reaction. Filter, extract with n-hexane (10 mL×3), combine the organic phases, dry and remove the solvent by rotary evaporation to obtain 2-methyl-2-pentanol.
[0041] (2) Dissolve 0.42 mol of anhydrous zinc chloride in 0.42 mol of concentrated hydrochloric acid, and cool for subsequent use. Add 0.4mol 2-methyl-2-pentanol, shake slightly, and take the organic phase to obtain 2-methyl-2-chloropentane.
[0042] (3) Add 30 mL of anhydrous tetrahydrofuran into a 100 mL three-necked flask, add 0.46 mol of sodium hydride in an ice bath, stir well, then add 0.4 mol of 1,1,2-ethanetricarboxylic acid triethyl es...
Embodiment 3
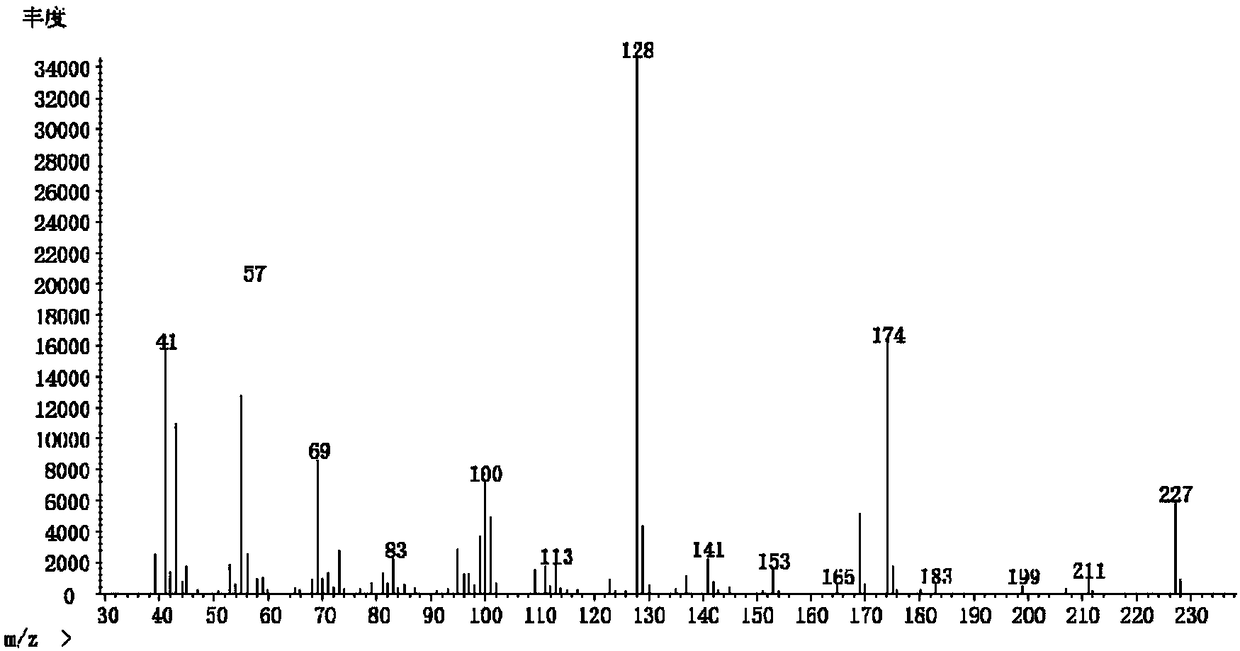
[0045] Example 3: 2-(1,1-dimethylpentyl)succinic acid
[0046] (1) Add 0.46 mol of acetone (re-distilled or add anhydrous magnesium sulfate to remove water) dropwise to 1-pentylmagnesium bromide in tetrahydrofuran under ice-bath conditions, and stir overnight at room temperature. Under ice-bath conditions, 0.50 mol of deionized water was added dropwise to quench the reaction. Filter, extract with n-hexane (10mL×3), combine the organic phases, dry and remove the solvent by rotary evaporation to obtain 2-methyl-2-hexanol.
[0047] (2) Dissolve 0.44 mol of anhydrous zinc chloride in 0.44 mol of concentrated hydrochloric acid, and cool for subsequent use. Add 0.4mol 2-methyl-2-butanol, shake slightly, and take the organic phase to obtain 2-methyl-2-chlorohexane.
[0048] (3) Add 30 mL of anhydrous tetrahydrofuran into a 100 mL three-necked flask, add 0.46 mol of sodium hydride in an ice bath, stir evenly, add 0.4 mol of 1,1,2-ethanetricarboxylic acid triethyl ester, and continue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com