Application method of catalysts capable of synthesizing cyclic carbonate under normal temperature and atmosphere
A cyclic carbonate, application method technology, applied in catalytic reactions, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of low catalyst activity and high cost, and achieve high activity. , low price, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
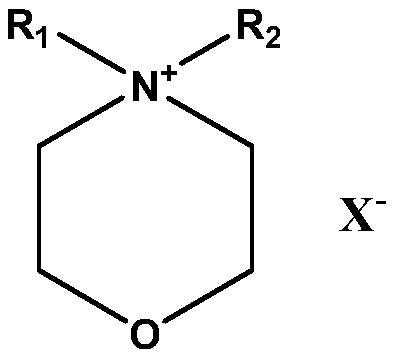
[0025]In a 50mL stainless steel autoclave, add 9.25g (0.1mol) of epichlorohydrin, 1.752g (0.005mol) of N-methyl-N-dodecylmorpholine ammonium bromide (the preparation refers to N-methyl -The preparation of N-hexadecylmorpholine ammonium bromide, only dodecyl bromide replaces hexadecane bromide) as the main catalyst, 0.84g (0.005mol) cobalt acetate is the cocatalyst, and the airtight reaction kettle is continued Introduce carbon dioxide, keep the pressure in the kettle at 0.1MPa, slowly raise the temperature to 35°C, react for 24 hours, cool to room temperature, slowly release the pressure, and distill the resulting liquid under reduced pressure to obtain the product 4-chloromethyl-[1, 3] The yield of dioxolan-2-one cyclic carbonate was detected by gas chromatography spectrometry and NMR, and the yield was 98.2%. The NMR data are as follows: 1 HNMR (CDCl 3 , 300MHz): δ (ppm): 3.78 (dd, J = 3.6, 12.3Hz, 1H); 3.86 (dd, J = 4.9, 12.3Hz, 1H); 4.44 (dd, J = 5.7, 8.8Hz, 1H) ; 4.64 ...
Embodiment 2
[0027] With the reaction conditions and detection method in embodiment 1, change main catalyst used into 1.61g (0.005mol) N-methyl-N-decylmorpholine ammonium bromide (its preparation is with reference to N-methyl-N-decyl ammonium bromide) The preparation of hexaalkylmorpholine ammonium bromide, only decane bromide is replaced hexadecane bromide), cocatalyst changes zinc bromide 0.225g (0.001mol), obtains 4-chloromethyl-[1,3 ] Dioxolan-2-one with a yield of 75.4%.
Embodiment 3
[0029] With reaction conditions and detection method in embodiment 1, change main catalyst used into 0.306g (0.001mol) N-methyl-N-dodecylmorpholine ammonium chloride (its preparation is with reference to N-methyl-N- -The preparation of hexadecylmorpholine ammonium bromide, only dodecyl chloride is replaced hexadecane bromide), promotor changes magnesium sulfate 0.012g (0.0001mol), obtains 4-chloromethyl-[1 , 3] Dioxolan-2-one, the yield was 86.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com