Pig ω7 interferon mutant and its preparation method and application
A mutant and interferon technology, which is applied in the field of preparation and application of swine ω7 interferon mutants, can solve problems such as affecting daily life, viral diseases affecting pig breeding industry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
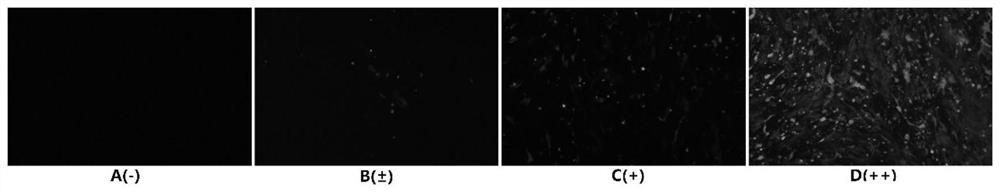
[0069] Example 1 Expression and detection of porcine ω7 interferon and its signal peptide mutant gene in silkworm bioreactor
[0070] 1. Experimental method
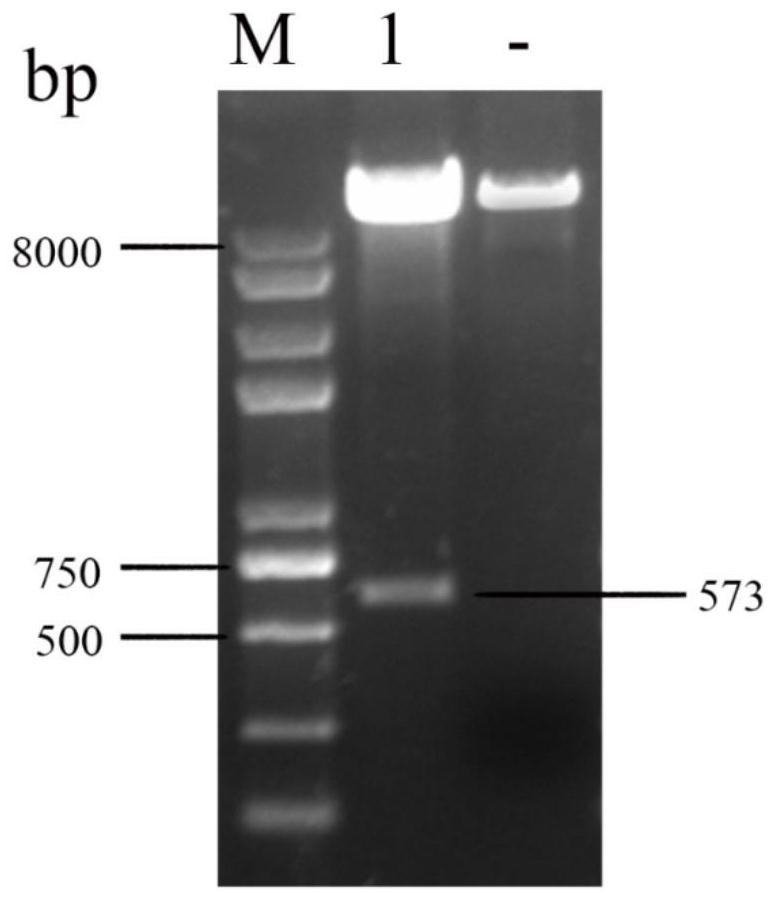
[0071] 1.1 Target gene synthesis and construction of recombinant plasmids
[0072] The present invention analyzes all porcine ω7 interferon amino acid sequences on NCBI, performs sequence comparison and signal peptide analysis, and finally determines that the amino acid sequence with the accession number ACF17568.1 is the original sequence, and its amino acid sequence is shown in SEQ ID NO.1 , the nucleotide sequence of its coding gene is shown in SEQ ID NO.2. During the sequence analysis, it was found that the signal peptide of this sequence had multiple cleavage peaks. In view of the previous discovery that the cleavage site of the signal peptide affects the secretion efficiency of interferon, the present invention decided to mutate the amino acid sequence of porcine ω7 interferon. Using SignalP4.1 to predict the sig...
Embodiment 2
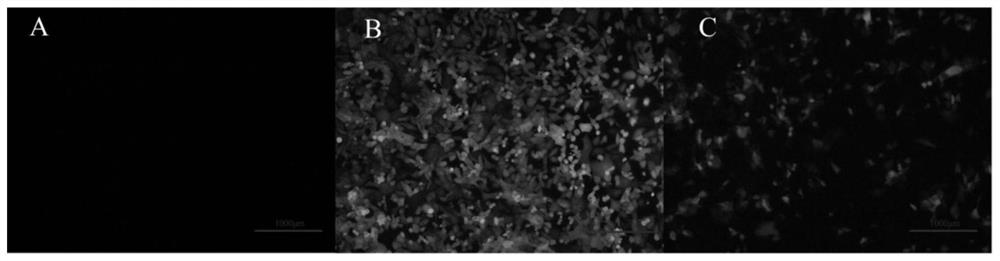
[0099] Example 2 Expression and detection of optimized SwIFN-ω7-S mutant in silkworm bioreactor
[0100] 1. Experimental method
[0101] 1.1 Construction of pig ω7-type interferon mutant gene
[0102] The present invention utilizes OptimumGene TM Technology optimized the above-mentioned porcine ω7 interferon mutants, modified the gene sequence according to the codon preference of the silkworm in the bioreactor, and improved the GC content, CpG dinucleotide content, CpG dinucleotide content, and Codon bias, mRNA secondary structure, mRNA free energy stability, RNA instability motif, repeat sequence and other related parameters are optimized and designed, which is conducive to improving the transcription efficiency and translation efficiency of the optimized gene in silkworm , and keep the final translated protein sequence unchanged.
[0103] In order to improve the translation initiation efficiency in the silkworm baculovirus eukaryotic expression system, the Kozak sequence ...
Embodiment 3
[0122] Example 3 Expression and Detection of SwIFN-ω7-S Mutant Optimization and Amino Acid Single Point Mutation in Bombyx mori Bioreactor
[0123] 1. Experimental method
[0124] 1.1 Construction of pig ω7-type interferon mutant gene
[0125] Based on the results of Example 2, the present invention uses the gene sequence of the codon-optimized SwIFN-ω7-S-O mutant as a template, and designs multiple pairs of primers to perform site-directed mutation on this sequence. The site-directed mutation is performed by fusion PCR. For the method of PCR, see "2. Experimental method" above.
[0126] The mutation sites are S27F, I33V, L61F, D67E, H70Q, A74T, I93T, K94E, D101N, I103T, H109C, Q114R, M138V, Q143E, H146R, I161T, E185V, H186D and P190S; The variant was named SwIFN-ω7-S-O-M (1-19) mutant.
[0127] Primers required for amino acid single-site, double-site and multi-site mutations in the nucleotide sequence of SwIFN-ω7-S-O mutant:
[0128] (1) Both upstream and downstream prime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com