A-site doping type double-perovskite catalyst as well as preparation method and application thereof
A double perovskite, doped technology, applied in chemical instruments and methods, heterogeneous catalyst chemical elements, physical/chemical process catalysts, etc., can solve the problem of limited perovskite, low specific surface area, poor characteristics, etc. problems, to achieve the effects of excellent thermal stability, high catalytic activity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
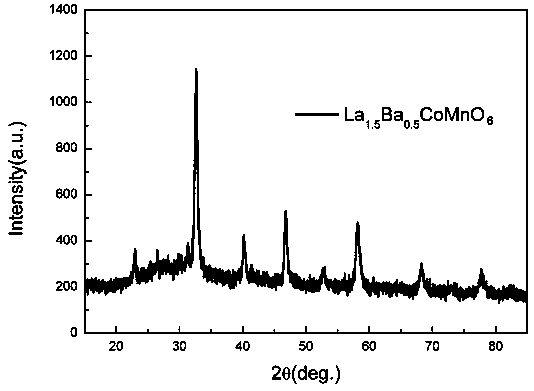
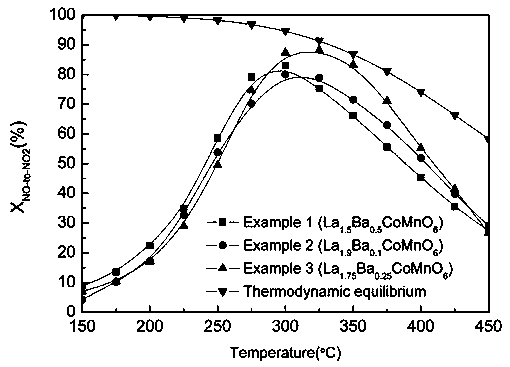
[0022] Example 1: In this example, the A-site doped double perovskite catalyst is La 1.5 Ba 0.5 CoMnO 6 ;
[0023] The preparation method of the A-site doped double perovskite catalyst, the specific steps are as follows:
[0024] (1) Add A salt (A salt is lanthanum nitrate), A' salt (A' salt is barium nitrate), B salt (B salt is cobalt nitrate) and B' salt (B' salt is manganese nitrate) Grinding in water and ethanol until dissolved to obtain ethanol metal salt solution, wherein the molar ratio of A salt (lanthanum nitrate), A' salt (barium nitrate), B salt (cobalt nitrate) and B' salt (manganese nitrate) is 1.5:0.5: 1:1;
[0025] (2) Mix the ethanol metal salt solution, potassium nitrate and sodium nitrate obtained in step (1), dry and volatilize the ethanol to obtain a mixed salt; wherein A salt (lanthanum nitrate), A' salt (barium nitrate), B salt (cobalt nitrate ) and B' salt (manganese nitrate) to the total molar ratio of potassium nitrate and sodium nitrate is 1:30; ...
Embodiment 2
[0030] Example 2: In this example, the A-site doped double perovskite catalyst is La 1.9 Ba 0.1 CoMnO 6 ;
[0031] The preparation method of the A-site doped double perovskite catalyst, the specific steps are as follows:
[0032] (1) Add A salt (A salt is lanthanum nitrate), A' salt (A' salt is barium nitrate), B salt (B salt is cobalt nitrate) and B' salt (B' salt is manganese nitrate) Grinding in water and ethanol until dissolved to obtain ethanol metal salt solution, wherein the molar ratio of A salt (lanthanum nitrate), A' salt (barium nitrate), B salt (cobalt nitrate) and B' salt (manganese nitrate) is 1.9:0.1: 1:1;
[0033] (2) Mix the ethanol metal salt solution, potassium nitrate and sodium nitrate obtained in step (1), dry and volatilize the ethanol to obtain a mixed salt; wherein A salt (lanthanum nitrate), A' salt (barium nitrate), B salt (cobalt nitrate ) and B' salt (manganese nitrate) to the total molar ratio of potassium nitrate and sodium nitrate is 1:30; ...
Embodiment 3
[0037] Example 3: In this example, the A-site doped double perovskite catalyst is La 1.75 Ba 0.25 CoMnO 6 ;
[0038] The preparation method of the A-site doped double perovskite catalyst, the specific steps are as follows:
[0039] (1) Add A salt (A salt is lanthanum nitrate), A' salt (A' salt is barium nitrate), B salt (B salt is cobalt nitrate) and B' salt (B' salt is manganese nitrate) Grinding in water and ethanol until dissolved to obtain ethanol metal salt solution, wherein the molar ratio of A salt (lanthanum nitrate), A' salt (barium nitrate), B salt (cobalt nitrate) and B' salt (manganese nitrate) is 1.75:0.25: 1:1;
[0040] (2) Mix the ethanol metal salt solution, potassium nitrate and sodium nitrate obtained in step (1), dry and volatilize the ethanol to obtain a mixed salt; wherein A salt (lanthanum nitrate), A' salt (barium nitrate), B salt (cobalt nitrate ) and B' salt (manganese nitrate) to the total molar ratio of potassium nitrate and sodium nitrate is 1:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com