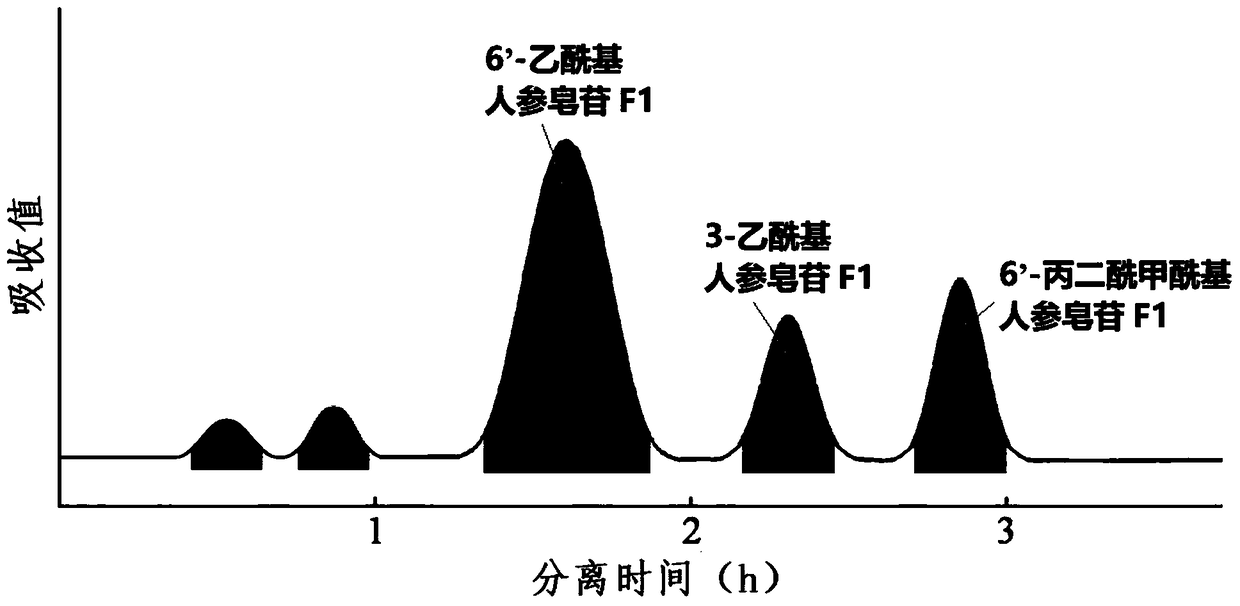
Method for preparation of 6'-malonyl formyl ginsenoside F1
A technology of malonylformyl human and ginsenoside, applied in the field of chemistry, can solve the problems of weak, inferior, and large loss of tyrosinase inhibitory activity, and achieve the effect of easy large-scale application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The following describes the essential content of the present invention in detail with reference to the drawings and embodiments, but the protection scope of the present invention is not limited thereto.
[0029] 1. Experimental materials and instruments
[0030] Dried ginseng flower buds were purchased from Bozhou Traditional Chinese Medicine Market.
[0031] LX-68 macroporous adsorption resin was purchased from Zhengzhou Qinshi Technology Co., Ltd.
[0032] The high-speed countercurrent chromatograph TEB300B was purchased from Shanghai Tongtian Biotechnology Co., Ltd.
[0033] 2. Experimental methods and results
[0034] 1. Preparation of total extract
[0035] Take 5 kg of dried ginseng flower buds, crush them with a pulverizer, and pass through an 80-mesh sieve to obtain ginseng flower bud powder. Then it was extracted with 95% ethanol at a 1:10 ratio of material to liquid and refluxed for 3 times for 2 hours each time. The extracts were combined and concentrated into a paste t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com