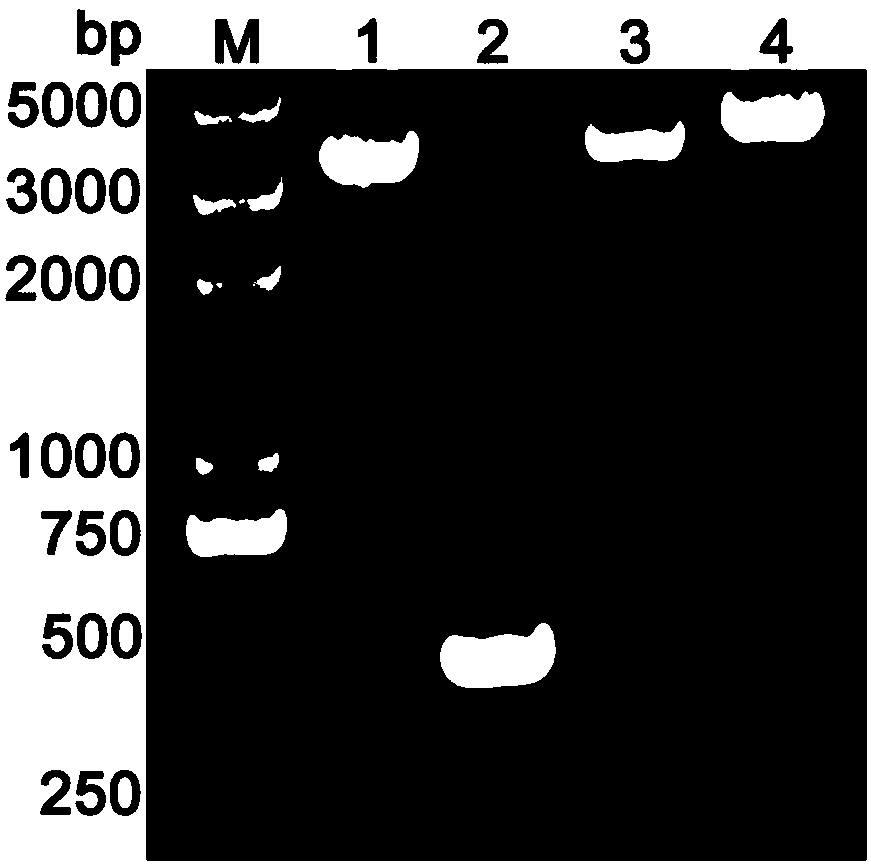
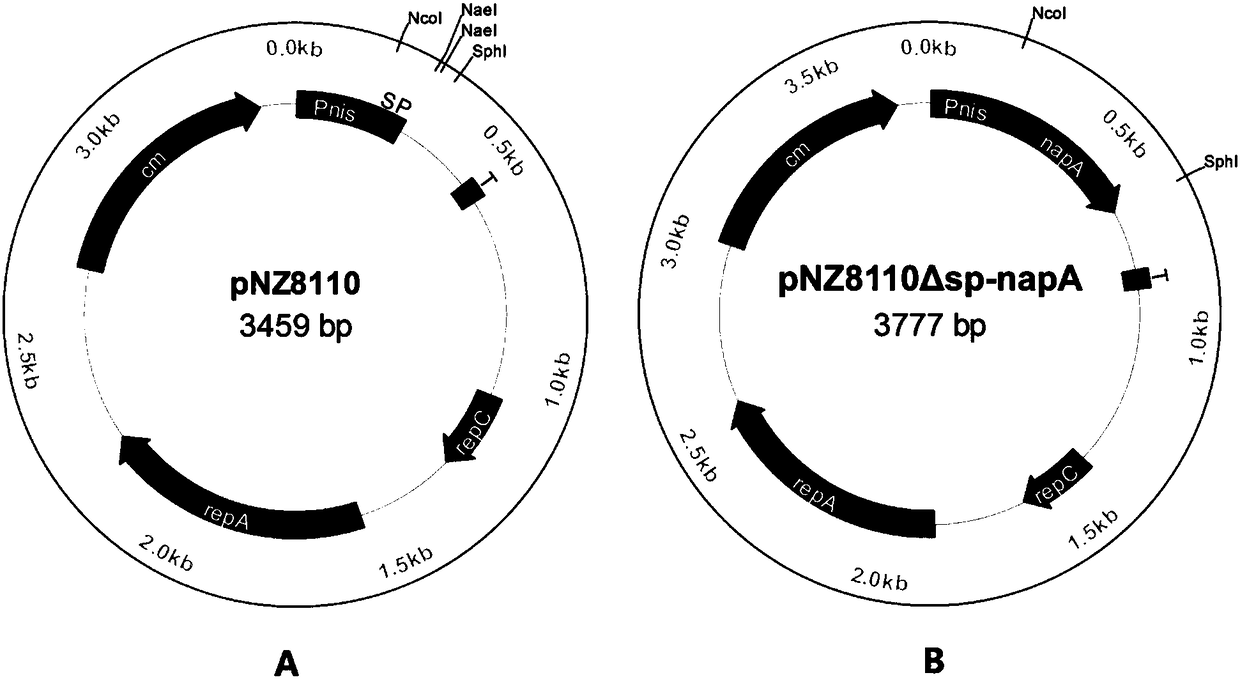
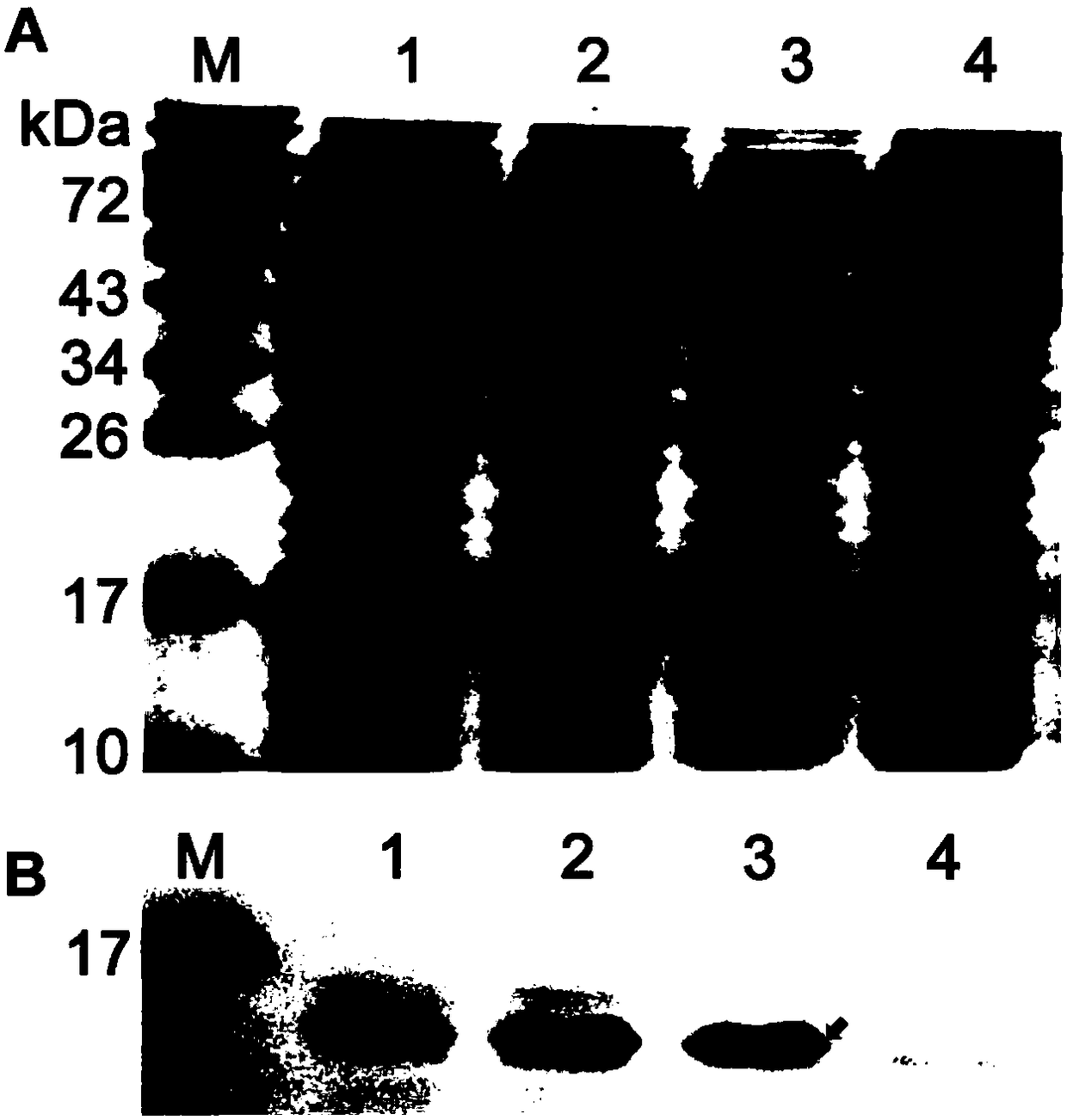
Helicobacter pylori NapA protein expressed recombinant vector, recombinant strain and preparation methods and application thereof
A Helicobacter pylori and recombinant vector technology, applied in the biological field, can solve the problems of NapA protein biological function influence, failure to be excised by bacteria, unusable and other problems, achieve obvious immune protection effect, obvious immune regulation effect, and improve production level. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Construction of recombinant vector
[0074] 1. Culture of Helicobacter pylori
[0075] Take out the Helicobacter pylori H.pylori MEL-HP27 (CGMCC NO.1338) strain from the refrigerator at -80°C, take 500 μl of the bacterial liquid and add it to the Brucella blood plate medium, spread the bacterial liquid evenly with a glass rod, and wait until the bacterial liquid is completely After the medium was absorbed, at 37°C, under microaerophilic conditions (5% O 2 , 10% CO 2 , 85%N 2 ) for 3 to 4 days.
[0076] 2. Extraction of Helicobacter pylori genomic DNA
[0077] (1) Scrape Helicobacter pylori cells cultured for 3-4 days into a 1.5ml centrifuge tube filled with 500μl deionized water, add 100μl 100g / L SDS solution, and boil for 5min.
[0078] (2) Add RNase to a final concentration of 50 μg / ml, 37°C for 1 hour, add proteinase K (final concentration 50 μg / ml), and act at 42°C for 1 hour.
[0079] (3) Use equal volumes of phenol, phenol chloroform (equal volume ...
Embodiment 2
[0093] Example 2 Construction of Lactococcus lactis recombinant strain
[0094] 1. Electrotransformation and screening of L. lactis NZ3900 strain
[0095] (1) Referring to the instructions of the authorized patent (ZL201210218281.9), culture L. lactis NZ3900 strain with GM17 medium, and prepare L. lactis NZ3900 competent cells.
[0096] (2) Take out the stored L.lactis NZ3900 competent cells from the -80°C refrigerator and put them on ice. Take 1 μl of the purified ligation product in Example 1 and add it to 40 μl L. lactis NZ3900 competent cells, mix well, and place on ice for 5 minutes.
[0097] (3) Add the bacterial solution to the 2mm electric shock conversion cup pre-cooled with ice; wipe off the water outside the electric shock cup, and put it into the electric shock instrument; set the electric shock parameters to 25μF, 200Ω, 2500V, and perform electric shock; Add 1ml ice-cold GM 17 MC recovery medium, mix well, transfer the bacterial solution to a 1.5ml EP tube, and...
Embodiment 3
[0174] Example 3 Application of L.lactis NZ3900 / pNZ8110Δsp-napA in the preparation of oral vaccines
[0175] Referring to Examples 1 and 2 and Test Example 1, the L.lactis NZ3900 / pNZ8110Δsp-napA strain was cultured in GM17 medium, the expression of the antigen NapA was induced with Nisin, and the bacterial solution was made into a dosage form suitable for oral administration by humans for use as an oral vaccine.
[0176] After oral administration of the vaccine, humans can develop immunity against Helicobacter pylori infection and have beneficial effects in preventing and treating Helicobacter pylori-related diseases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com