Porous nitrogen-doped carbon supported cobalt nano-material, preparation method and application thereof
A technology of nitrogen-doped carbon and porous materials, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of poor durability and expensive electrocatalysts, and achieve good electrocatalytic performance, The effect of excellent electron transport performance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
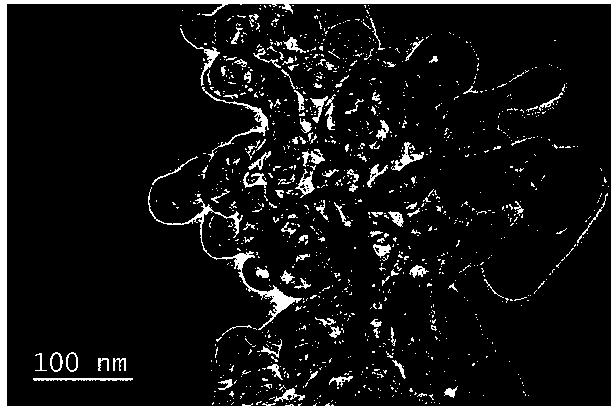
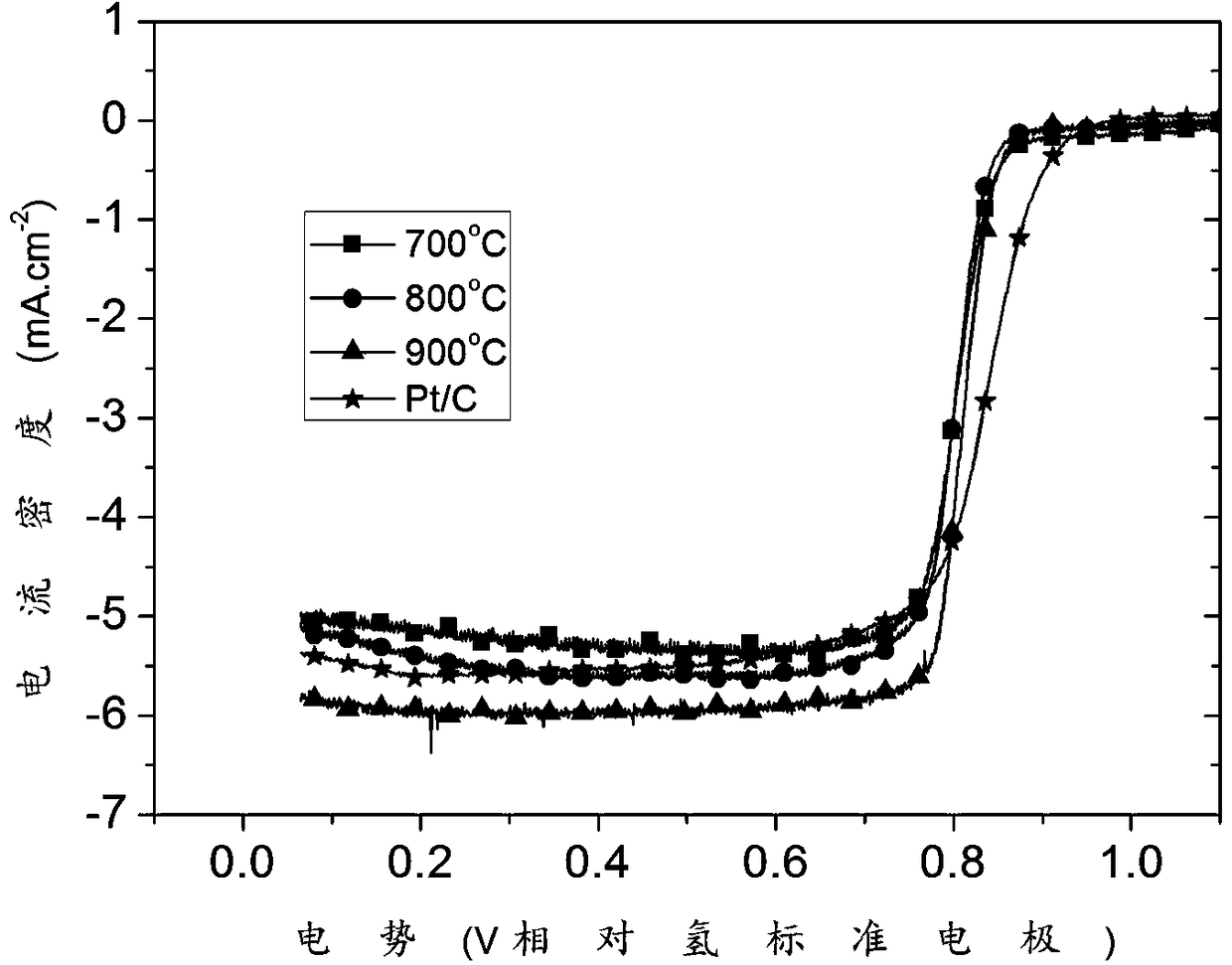
[0048] (1) Take 264.6 mg Na 3 C 6 h 5 o 7 2H 2 O was dissolved in 20 ml deionized water, and 174.6 mg Co(NO 3 ) 2 ·6H 2 O stirred to dissolve, as A solution. Take another 132.9 mg K 3 [Co(CN) 6 ] was dissolved in 20 ml deionized water as solution B. After both solution A and solution B are fully dissolved, pour solution B into solution A under stirring, stir for 2 minutes, and leave to age for 24 hours. After that, put the obtained precipitate into a centrifuge tube, centrifuge for 3 minutes, add deionized water to wash 3 times, and set the rotation speed to 10000 rpm·min -1 、9500rmp·min -1 、9000rmp·min -1 . The sample obtained by centrifugation was placed in a vacuum drying oven and dried at 60 °C for 12 h to obtain a cobalt-based precursor. The morphology scanning image is as follows: figure 1 shown.
[0049] (2) Weigh 250 mg of Tris and dissolve it in 100 ml of deionized water to make a buffer solution. Dissolve 443.8 mg of the cobalt-based precursor obtained...
Embodiment 2
[0053] (1) Take 1323.5mg Na 3 C 6 h 5 o 7 2H 2 O was dissolved in 50 ml deionized water, and 873.9 mg Co(NO 3 ) 2 ·6H 2 O stirred to dissolve, as A solution. Another 738.5mg K 3 [Co(CN) 6 ] was dissolved in 50 ml deionized water as solution B. After both solution A and solution B are fully dissolved, under stirring, inject solution B into solution A with a syringe pump at an injection speed of 2m·min -1 , and stirred for 24 h after injection. After that, put the obtained precipitate into a centrifuge tube, centrifuge for 3 minutes, add deionized water to wash 3 times, and set the rotation speed to 10000 rpm·min -1 . The centrifuged sample was placed in a vacuum oven and dried at 60 °C for 14 h to obtain a cobalt-based precursor.
[0054] (2) Weigh 250 mg Tris and dissolve it in 100 ml deionized water to make a buffer solution. Dissolve 378.2 mg of the cobalt-based precursor obtained in step (1) in the buffer solution, stir, and sonicate to make it evenly mixed. I...
Embodiment 3
[0058] (1) Take 529.4 mg Na 3 C 6 h 5 o 7 2H 2 O was dissolved in 40 ml deionized water, and 349.2 mg Co(NO 3 ) 2 ·H 2 O stirred to dissolve, as A solution. Another 265.8 mg K 3 [Co(CN) 6 ] was dissolved in 40 ml deionized water as solution B. After both solution A and solution B are fully dissolved, pour solution B into solution A under stirring, stir for 3 minutes, and leave to age for 24 hours. After that, put the resulting precipitate into a centrifuge tube, centrifuge for 3 minutes, add deionized water to wash 3 times, and rotate at 9500rmp·min -1 . The centrifuged sample was placed in a vacuum oven and dried at 60 °C for 10 h to obtain a cobalt-based precursor.
[0059] (2) Weigh 250 mg of Tris and dissolve it in 100 ml of deionized water to make a buffer solution. Dissolve 300 mg of the cobalt-based precursor obtained in step (1) in the buffer solution, stir and sonicate to make it evenly mixed. In addition, 150 mg of dopamine hydrochloride was dissolved in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com